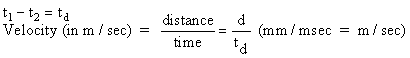
In 1850,
Hermann von Helmholtz first estimated the velocity of nerve impulses transmitted
in a frog nerve-muscle preparation with a mechanical kymograph and writing
levers. During the latter half of the 19th century, concepts underlying
the modern theory of nerve conduction were developed by Sherrington and others,
but modern electro-physiological research awaited the development of the cathode
ray oscilloscope. Using this apparatus, Erlanger and Gasser in 1921 first
measured the ionic currents of compound action potentials. Their studies
provided an important foundation for our present understanding of nerve
function. The frog sciatic nerve was the classical preparation for study
of the action potential until experimental researchers developed intracellular
recording methods for studying squid giant fibers.
Action
potentials can be elicited simultaneously in thousands of axons of a peripheral
nerve like the sciatic by electrical stimulation. The collective response
is termed a compound action potential. Indiscriminate as this gross
recording technique may seem, some basic aspects of neuronal conduction--maximal
firing rates, threshold, conduction velocity, and the role of axon size and
myelination--can be demonstrated using the whole nerve approach.
Preliminary setup: It is essential to familiarize yourself with the instruments and recording system before beginning the dissection. The instrumentation may seem formidable, but generally it is the faltering viability of a biological preparation that ends the experiment. Therefore, any practical or conceptual problems regarding the equipment should be cleared up before the experimental animal is touched.
Surgical
procedure: You will be provided with a doubly-pithed Bullfrog (Rana
catesbiana). Pick up a fold of skin at midabdomen with forceps and, avoiding
cutting into the abdominal cavity with your scissors, cut the skin all the way
around the frog. Pull the skin down,
Separate the
muscles and free the nerve from surrounding tissue using blunt glass tools
whenever you must touch the nerve. Make such a tool by heating a glass rod
in a bunsen burner flame and pulling out a working tip smoothed to about the
width of a dulled pencil lead. Apply amphibian perfusion fluid (Frog
Ringer's solution) liberally as you work.
Hold the
urostyle up, and carefully cut the muscles on both sides of the bone. Free
the caudal end of the urostyle and lift it up to expose the underlying
structures. Note the two regions of white fibers that compose the sciatic
nerve plexus. Each sciatic nerve originates as three spinal nerve roots.
Cut the urostyle at its hinge. Carefully tie the roots together with the
end of a 10 cm length of Ringer's-soaked cotton thread. Cut the nerve
roots as close to the spinal cord as possible. Now free the nerve from the
hip to the knee, lifting with the thread as necessary. [CAUTION: Do
not stretch the nerve!] When the nerve has been totally freed, cut through
the distal end with scissors. Immerse the nerve in a small beaker of
perfusion fluid.
Installing
the nerve in the recording apparatus: Plug any holes in the nerve
chamber with Vaseline, and fill the chamber with perfusion fluid to a point
about 5 mm above the electrode wires. Lay the nerve lengthwise in the
chamber so that it floats above the wires. Note which end of the nerve is
which (anterior end is thicker). Manipulate the nerve with glass tools as you
draw off enough of the fluid so that it comes to rest on the electrode wires.
The nerve must be in physical contact with each of the wires, and the level of
the fluid must be well below all of the wires to prevent them shorting out.
One end of the nerve may remain in the fluid, but not both. Place the
cover over the nerve chamber to prevent drying.
If drying of
the nerve tissue seems to be a problem, add a layer of mineral oil saturated
with Ringer's atop the fluid already in the chamber. Cover the electrodes
and the nerve. As oil is added it may lift the nerve off the electrodes.
To prevent this, add the oil/Ringer's mixture by dropping it over and on top of
the nerve until the nerve is immersed. Look to see that good contact is
made between the nerve and each electrode. If contact between the nerve
and electrode wires is lost, it can be reestablished by manipulating the nerve
using a dropper and a half-squeezed-out drop of Ringer's solution.
Analog
recording procedure: Arrange the electrode leads so that you stimulate
and record at opposite ends of the nerve and ground the center (See Fig. 2).
For recording, connect a pair of cables to two electrodes near the distal (thin)
portion of the nerve and connect the other end of this pair of cables to the
input of the preamplifier [or direct to the oscilloscope if preamplifiers are
not used]. These cables should be as short as possible to minimize the
pickup of electrical interference.
Connect
a third wire (green if possible) to one of the other electrodes about midway
along the nerve and run it to a ground terminal on the preamplifier.
Connect another pair of cables from the stimulator output to a pair of
electrodes at the proximal (thick) end of the nerve. Make sure that the
negative electrode is nearest the recording electrodes. The action
potential is initiated at the negative electrode (cathode).
The
presence of the anode (positive electrode) between the cathode and recording
electrodes may block AP transmission since the anode hyperpolarizes the nerve.
Connect
the output of the preamplifier to the input of the oscilloscope with appropriate
shielded cables and connectors. Connect the stimulator sync out (trigger
output) to the trigger input of the oscilloscope. This arrangement
synchronizes the initiation of the oscilloscope sweep with the output pulse of
the stimulator. Refer to Fig. 3 for the recording setup.
Use
the following initial settings on your equipment:
| Stimulator | Preamplifier |
| Frequency 7/sec | Gain 100X |
| Duration 0.1 msec | Low band pass filters = 10 Hz |
| Voltage 0.1 V to start | Low |
| Mode off at first | High band pass filters = 3-5 kHz |
| Input on USE |
| Oscilloscope | ||||||||
|
Calibration:
Adjust the overall system gain (preamplifier plus oscilloscope) to about 100 mV/div.
Check by using the preamplifier calibration function. Set the Grass preamp
input knob to CAL 100 mV and depress the G1 NEG
button several times in succession. Alter the oscilloscope vertical
amplifier gain so as to produce a 1 cm deflection when G1 is pulled NEG.
During the experiments, you may need to alter the system gain to best display
the compound AP's that the nerve produces, and you should recalibrate using this
approach when doing so.
Recall
that these are suggested settings for starting the experiment. Readjusting
the gain of the vertical amplifier and the time base of the oscilloscope to
visually display the nerve action potential is an ongoing process. Just
keep accurate notes on the settings used whenever you record a piece of data.
Digital
recording with MacLab: Turn the MacLab and Macintosh on. Open
the folder for your lab group by double-clicking on the icon. This folder
should contain all the software you will need to run and analyze today's lab.
Run the program called SCOPE by double-clicking on the icon labeled
"Sciatic Nerve Lab". This will launch SCOPE and provide you with
a ready-to-record computerized oscilloscope. This digital oscilloscope
differs from the Kikusui in several ways, but most important for us is the
MacLab's ability to record and store a waveform for analysis.
Plug
in the Kikusui oscilloscope's CH 1 output (on the back of the machine) to the
MacLab's input CH 1. With the Kikusui on and free-running (trigger = auto)
but the stimulator MODE control off and the nerve quiescent, open the MacLab
input amplifier dialog box in SCOPE (just point and click with the cursor).
With
the Grass preamplifier input knob on CAL 100 mV, hit
the G1 NEG button on the preamp several times. This should produce a 1 cm
deflection on the oscilloscope (since you've already calibrated the overall
system gain up to that point) and a also should produce a good sized square wave
("good-sized" being about 1/3 full scale or so) on the SCOPE input
amplifier recording trace. Reset the oscilloscope vertical amplifier gain
or the SCOPE input amplifier gain (click and drag with the cursor) to give an
easily visible wave on the computer when G1 NEG is pushed. This CAL value
from the Grass preamp can be used later to calibrate the computer recordings, so
once you've started, record a CAL wave or two of known size to go along with
your recorded nerve action potentials. When you're satisfied, close the
SCOPE input amplifier box by clicking on OK.
Plug
the stimulator trigger output into the MacLab trigger input using the “pulse
stretcher” box. Check the display dialog box in SCOPE to examine the
trigger settings under recording. This should be set for external.
Note the recording settings too--multiple gets really busy really fast, so you
probably should use single sweeps or overlay mode at first to record.
When
you're ready to record a wave from your nerve preparation (later, not yet!), you
will set up the oscilloscope to sweep and display the AP. When SCOPE is
triggered, manually with the mouse (USER), or by the stimulator (TRIGGER), a
wave will appear on the screen. You can record the displayed wave or
choose New Data to record another one. Practice using the SCOPE recording
feature without recording AP's until you've got it down. Once recording,
note your stimulus voltage and other data on the comments notebook attached to
each scope “page”. Record a calibration pulse to use in measuring the
size of the AP's. See the SCOPE instruction manual for more detail.
Now
that you have reached a good understanding of the equipment's setup and
operation, you're finally ready to start the experiments. Read through
each section in advance and know what the goal of that experiment is before you
begin.
1.
Threshold: First, activate the stimulator by placing the output mode
switch in the continuous (multiple) position. Gradually increase the
stimulus voltage from 0.1 V. You will see the stimulus artifact as the
first-appearing wave; this is the stimulating voltage conducted on the outside
of the nerve and picked up through the recording electrodes. The artifact
can be seen to vary with stimulus duration.
Continue
to increase the stimulus voltage until a second wave appears to the right of the
artifact. This is the compound action potential. Continue to
increase the stimulus until this wave reaches a maximum amplitude. Reduce
the voltage and note the voltage at which the AP first appears. This is
the threshold voltage for the most sensitive axons (or those most accessible to
the stimulating current).
Increase
stimulus intensity until a maximal response is seen. At this point all the
nerve fibers are actively conducting AP's and the waveform seen is the sum of
all of them. This growth of the AP with increasing stimulus intensity
obscures the fact that the action potential of each individual fiber is an
all-or-none event. The compound AP has these distinguishing properties:
It is not the first deflection observed; its amplitude, though initially
increased by raising stimulus intensity, is not a linear function of stimulus
strength; its duration is not a direct function of stimulus duration; it does
not have the shape of the stimulus artifact.
Record
threshold and the voltage needed to recruit a maximal response. Record a
typical waveform with Scope in MacLab. Write down all instrument settings
and check the timebase and vertical calibration for your recording. Turn
off the stimulator to allow the nerve to rest.
2.
Recruitment of nerve fibers: To produce a graphical illustration of the
response of your nerve to different stimulus intensities, record several waves
at differing stimulus voltages between threshold and maximal voltage.
3.
Waveform--monophasic and biphasic: The shape of the waveform observed on
the oscilloscope screen depends on a number of factors. The distance
between recording electrodes, sweep rate, gain, filter settings, and condition
of the nerve all influence the shape of the compound AP observed.
Return
stimulus voltage to 0.1 V. Turn up the intensity until the volt-age is
about 10% above that needed to elicit a maximal response. Reverse the polarity
of the recording electrodes, if necessary, so that the initial deflection of the
displayed waveform is upward. The compound AP from an undamaged nerve is
usually biphasic. As the AP sweeps by the first recording electrode, it
drives that electrode negative with respect to the more distant electrode.
If you previously arranged the electrodes as above, the initial deflection will
be upward. Then, as the wave of depolarization (the AP) arrives at the
second recording electrode, making it negative, the oscilloscope trace is
deflected downward. Record the biphasic wave in Scope, recording
amplification and timebase for reference.
4.
Conduction velocity: Measuring the time and distance between appearance of
the AP at different recording electrodes can provide an estimate of the speed of
nerve AP conduction. Rearrange electrodes so that you stimulate at the
distal (thin) end and record at the proximal (thick) end. Determine
conduction velocity by moving the active (first) recording electrode and
recording time and distances as in Fig. 4. Measure times from the start of
the stimulus artifact (or beginning of the sweep) to the peak of the AP.
You can read the oscilloscope display most accurately if you spread it out with
a fast sweep speed. Measure distance traveled using a micrometer.
You can do this after you've finished if you're certain to record the electode
numbers used. Express conduction velocity in meters/second.
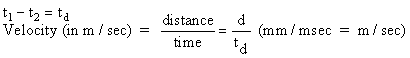
If
the nerve is very short, the conduction velocity may be estimated by measuring
the time interval between the beginning of the stimulus and the distance between
stimulating cathode and the first recording electrode. This measure is
less accurate because it includes an unknown time to initiate the impulse.
The accuracy of this method is increased by using a supramaximal stimulus
intensity and as brief a stimulus duration as is feasible.
5.
Fiber groups: Within the total population of fibers in frog sciatic nerve
there are several groups of axons of similar diameter and therefore, similar
threshold and conduction velocity. Connect the electrodes for monophasic
recording. Reduce the frequency to 5 stimulations/ sec. Try to
identify as many peaks of the compound AP as possible, by slowly increasing the
stimulus voltage and looking for the addition of new peaks. It should be
possible to find two of the three major peaks of the compound AP demonstrated by
Erlanger and Gasser (1968): A, the largest, corresponding to large
myelinated fibers; and C (the slowest wave), corresponding to very fine
unmyelinated fibers. Within the A wave you may be able to separate several
subpeaks, the A-alph, A-beta, and A-delta fibers (see Fig. 5).
The
conduction velocity of the C fibers is only 1/100 that of the A-alpha peak and
to see the C peak you must therefore stimulate at a low enough rate for it to
appear before the next A wave. The sweep rate should also be low (ca. 50
msec/div) and the stimulus intensity high.
Determine
the relative amplitudes, thresholds, and conduction velocities of each group of
fibers in your preparation. Fiber diameter is probably the most important
determinant of conduction velocity, with large fibers conducting faster.
6.
Strength-duration curve [optional]: The ability of the stimulus to elicit
a response is dependent on the stimulus duration as well as its intensity.
In other words, a response can be obtained using strong current for a short time
or a weak current for a long time. The relationship between strength and
duration can be determined empirically for your sciatic nerve preparation.
Vary
the duration and measure the threshold voltage. You may define threshold
as a small but observable response (for example, a 1 cm deflection). Use a
constant criterion for recording the threshold stimulus. Start by setting
the stimulus duration to 100 msec and gradually increase the stimulus intensity
until a response in noted. Decrease the duration to 50 msec and advance
the voltage until an identical response is seen. Continue this process for
a number of different stimulus durations.
Plot
a strength duration curve, with stimulus intensity (V) on the ordinate and
duration (msec) on the abscissa. Your curve should look approximately like
Fig. 6. The minimum intensity which elicits a response at infinite
duration is called the rheobase. Chronaxie (2X rheobase) is a measure of
the excitability of nervous tissue. The smaller its value, the more
excitable the nerve. These concepts have lost some of the importance they
once had for understanding nerve function, but chronaxie is still useful to
compare excitability of nerve and muscle tissues. Strength-duration curves
have also been used experimentally to follow the course of nerve and muscle
regeneration.
Record
and tabulate values for threshold, voltage for maximal response, conduction
velocity. Estimate conduction velocity and fiber diameter for different
fiber groups. Compare compound and single-cell AP's and mono- and biphasic
waves. Include your digital recordings of observed AP's, with time and
vertical scales identified.
Graph
response (peak height or mV) vs. stimulus intensity in part 1. Graph
stimulus intensity at threshold or a fixed response vs. duration and determine
chronaxie and time constant, if you obtained those data in the optional SD curve
experiment.
In
your report, discuss the main features of nerve action potentials, including the
ionic basis of the AP wave and its propagation. Explain how conduction
velocity varies in different animals [see Prosser 1973; Schmidt-Nielsen 1978;
Bullock, Orkand, and Grinnell 1978, all in the lab].
Aidley,
D.J. 1991. The physiology of excitable cells. 3rd ed. Univ. Press, Cambridge.
Baker,
P.F. 1966. The nerve axon. Sci. Am. 214:74-82.
Cragg,
B.G. and P.K. Thomas. 1957. The relationships between conduction velocity and
the diameter and internodal length of peripheral nerve fibers. J. Physiol.
136:606-614.
Erlanger,
J. and H.S. Gasser. 1930. The action potential in fibers of slow conduction in
spinal roots and somatic nerves. Am. J. Physiol. 92:43-82.
Erlanger,
J. and H.S. Gasser. 1968. Electrical signs of nervous activity. 2nd ed. Univ.
Pennsylvania Press, Philadelphia.
Erlanger,
J., H.S. Gasser, and G.H. Bishop. 1924. The compound nature of the action
current of nerve as disclosed by the cathode ray oscillograph. Am. J.
Physiol. 70:624-666.
Hill,
A.V. 1936. The strength-duration relation for electric excitation of medullated
nerve. Proc. Roy. Soc. B. 119:440-453.
Oakley,
B. and R. Schafer. 1978. Experimental neurobiology: a laboratory manual. Univ.
Michigan Press, Ann Arbor.
Stevens,
C.F. 1979. The neuron. Sci. Am. 241:54-65.