AB16. Relative Conditions and pKa
Reactivity is strongly affected by the environment around the molecules that are reacting. Usually the environment is a solvent; a solvent is a liquid in which the molecules are dissolved. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity.
Solvation is very important. When molecules are dissolved in a liquid, they can move around easily and mix with other molecules. That ease of motion facilitates reactions. In contrast, molecules in the solid state hardly move at all. Solid state reactions are very, very slow because molecules can't easily come into contact with each other. If two solids are mixed, molecules on the surface of the solid grains may react, but the molecules buried inside will be left untouched.
In order for one compound to dissolve in another, some intermolecular attractions must be present. In order to maximize these interactions, the solvent molecules probably need to arrange themselves somehow.
This organization of solvent molecules becomes even more important when ions are involved than when neutral molecules are dissolved. When ions are dissolved, anions are separated from cations. Solvent molecules must be able to interact with the ions so as to mitigate against the energetic costs of charge separation.
In organizing around a solute molecule, the usual interactions between the solvent molecules are disrupted. The solute molecule occupies a gap in the solvent molecules. For example, if the solvent is water, there must be a break in hydrogen bonding between water molecules to allow for the solute to swim among the waters.
Earlier we saw that larger atoms can more easily accommodate charge. This rule does not extend to the size of molecules. A larger molecular ion is generally not as easy to solvate as a small one. Larger ions require much more organization of solvent molecules. In addition, interactions between the solvent molecules (generally very favourable in solvents such as water) must be given up so that solvent molecules can move away from each other to open up space for the guest ions.
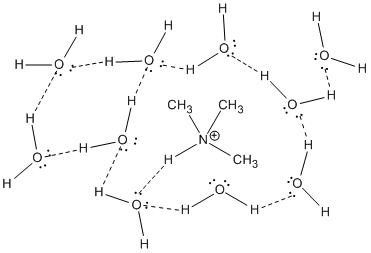
Figure AB16.1. Solvation of the relatively small trimethylammonium ion.
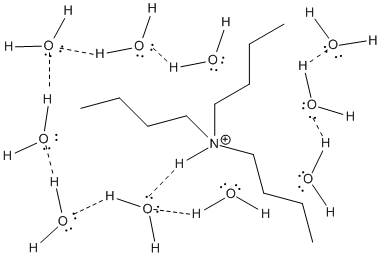
Figure AB16.2. Solvation of the relatively larger tributylammonium ion.
-
The size of a molecular ion being accommodated in a solvent matrix has an effect on the ion stability.
Different solvent effects arise partly because of different intermolecular attractions available to solvent molecules. Some solvents are hydrogen bonding. Water is a very common example, as are alcohols such as methanol. Others are only hydrogen bond-accepting, but not hydrogen bond-donating. Acetone and acetonitrile are examples.
Some solvents may more efficiently stabilize anions that form when Bronsted acids are deprotonated. As a result, pKa values may be different when measured in different solvents. For example, the pKa of water is reported as 15.7 in pure water, but when dissolved in DMSO it is reported as 32. Water is much less acidic in DMSO than when it is pure.
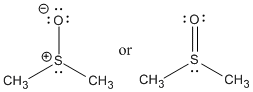
Figure AB16.3. DMSO is a very polar, aprotic solvent.
DMSO has something in common with water. Water is very polar, with a polar O-H bond. DMSO is very polar, having a strongly polarized S-O bond. Water can hydrogen bond. DMSO is capable of accepting hydrogen bonds. That means DMSO can use its lone pairs to donate to protons on other molecules. However, DMSO is aprotic. It does not have very positive hydrogens that can participate in hydrogen bonding. That means one DMSO molecule cannot hydrogen bond to another DMSO molecule like water can. As a result, it is not able to donate hydrogen bonds to anions, as water can. Water is therefore more able to stabilize anions, so molecules can ionize more easily in water than in DMSO.
-
Hydrogen bonding can be important in anion stabilization.
There is another reason to be aware of solvent effects in proton transfer reactions. Sometimes, solvents can themselves become involved in acid-base reactions.
For example, water is a very weak acid, but it can give up a proton. When it does so, it forms a hydroxide ion. Water could give its proton up to another anion if that anion could bind a proton more tightly than could hydroxide.
An example of this situation would occur if sodium amide were dissolved in water. Ammonia binds its proton more tightly than water. Thus, if sodium amide were dissolved in water, it would immediately become ammonia, removing a proton from water and forming sodium hydroxide.
As a result, the pKa of ammonia could not easily be measured in water because its conjugate base does not really exist in water. Assessing a pKa requires comparing how much of a compound remains protonated and comparing it to how much of the compound ionizes. As it turns out, the pKa of ammonia is around 41. That is high enough that this value was not determined directly. Instead, it had to be extrapolated by comparison with other data.
-
Sometimes, solvent participate in reactions. They are not always innocent bystanders.
Problem AB16.1. Explain why ammonium bromide, NH4Br, is more soluble in water than is sodium bromide, NaBr.
Problem AB16.2. The pKa of hydrogen cyanide, HCN, is about 13 in DMSO. Predict qualitatively how the pH would change if measured in a) water or b) pentane, CH3CH2CH2CH2CH3.
