SC19. Chiral catalysts for production of enantiomerically pure compounds
Chiral metal complexes became very important over the last 30 years of the twentieth century, particularly as companies sought to synthesize rare and difficult-to-obtain natural products for use as pharmaceuticals.
One of the landmark developments in this area was the production of chiral metal phosphine complexes. Phosphines are molecules containing phosphorus that bind to metal ions and promote catalytic activity. Among other workers, William Knowles of Monsanto was able to isolate complexes with chiral phosphorus atoms and use them to catalyze reactions producing almost entirely one enantiomer. The resulting synthesis of the anti-Parkinson's drug L-DOPA produces 97% of the desired enantiomer and only 3% of the opposite enantiomer.
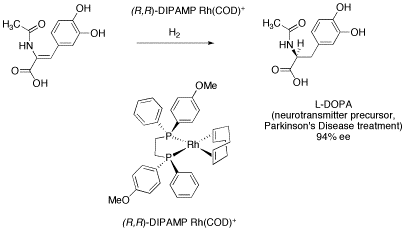
Figure SC19.1.. Knowles' catalyst in Monsanto's L-DOPA synthesis.
Go to Animation SC19.1. A three-dimensional model of Knowles' chiral catalyst.
Chiral catalysts work because metal-catalyzed reactions require the substrate to bind to the metal ion. If there is a chiral center at or very close to the metal ion, very often a substrate is forced to bind to the metal ion in a very specific way. In the case of the Monsanto L-DOPA synthesis, only one face of the C=C double bond can be presented to the metal atom (it won't fit together the other way), and the metal delivers two hydrogen atoms to that face of the double bond and not to the other.
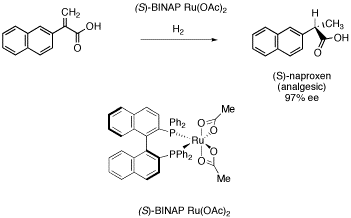
Figure SC19a. Noyori's catalyst in synthesis of (S)-naproxen.
Go to Animation SC19.2. A three-dimensional model of Noyori's chiral catalyst, (R)-configuration.
Go to Animation SC19.3. A three-dimensional model of Noyori's chiral catalyst, (S)-configuration.
Another pharmaceutical produced in highe enantiomeric excess employs a chiral metal complex that contains no chiral center. Ryoji Noyori is a professor at Nagoya University in Japan and also president of RIKEN, a major Japanese research institute involving about 3000 scientists. Among numerous other scientific contributions for which he is responsible, Noyori developed a number of complexes using BINAP ligands, which contain large, planar rings.
This case is roughly analogous to enantiomeric octahedral complexes like Co(en)33+, in which the the planes of the BINAP can twist in either one direction or the other. The rings cannot twist past each other, so when the BINAP complexes to the ruthenium, they get locked into either of two enantiomeric shapes, despite the fact that the compound doesn't really have a chiral center.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.