SC14. Optical resolution
Optical resolution refers to the separation of two different enantiomers from each other. Very rarely, this has been done if the crystalline forms of two enantiomers are visibly different from each other. For example, Louis Pasteur was able to separate the two enantiomers of tartaric acid from each other because the two compounds just happened to crystallize separately. The crystals that formed had multifaceted habits that were visibly mirror images of each other. He painstakingly separated the two enantiomers using a pair of tweezers and a microscope.
Most of the time, we don't get so lucky. Two enantiomers are normally so similar to each other physically that we can't separate them.
However, there can be a pressing need to do so. That's because two enantiomers may have identical physical properties, but they may have very different biological properties. In the cell, they may encounter other chiral materials, including proteins and sugars. Their interactions with those chiral molecules may be strongly influenced by how they fit together. The result may be the difference between a compound relieving a child's athsma or making it worse.
Chiral compounds, of course, are often compared to hands. Two enantiomers are like a right hand and a left hand, mirror images of each other that look exactly the same but are instead completely opposite.

Figure SC14.1. Handedness or chirality: a left and a right hand.
(You don't like hands with three fingers? Start reading the comics page. It's a well-established convention.)
The fact that the interaction of chiral compounds with other chiral compounds is influenced by their stereochemistry is often compared to the fit of your two hands to the same glove. Not surprisingly, one hand fits the glove better than the other one.

Figure SC14.1. Handedness or chirality: a left and a right hand, and one glove.
One way to separate two enantiomers is through chiral column chromatography. Chiral column chromatography uses something like a hand-glove approach to separate two enantiomers from each other.
In case you aren't familiar with chromatography, we should take a look at it in general terms first. Chromatography describes the separation of compounds through their different physical interactions between two different phases as the compounds pass through a long column or tube.
The column is packed with one material, called the stationary phase, whose job it is to stick to the compounds and keep them from moving through the tube. The stationary phase could be simple alumina or silica (like very finely powdered sand) or it could be a complicated synthetic material protected by a patent.
Another material, called the mobile phase, flows through the column. The job of the mobile phase is to tug the compounds along and get them through the column. The mobile phase could be a gas, allowed to flow through the column from a compressed tank, or a liquid, pushed through with a pump.
A tug-of-war ensues between the mobile phase and the stationary phase. Different compounds will have different affinities for each phase. Compounds that are more strongly attracted to the mobile phase will move more quickly. Compounds that are held more firmly by the stationary phase will move more slowly.
Chiral column chromatography involves the use of one of those patented stationary phases. The stationary phase has a specific kind of chiral molecule (or a chiral moiety, just a piece of a molecule) bonded all over it. The differing interactions between the two enantiomers and the chiral elements of the stationary phase result in the two compounds moving through the column at different rates. The compound that fits together with the chiral stationary phase will slow down and take longer to come through the column.
A second major way of separating two enantiomers is through the use of a chiral resolving agent. A chiral resolving agent is a compound that already has a chiral centre of its own. It is used in its pure form: only one enantiomer and not the other, having already been purified somehow (or selectively synthesized). This compound has to be able to react somehow with the target compound. When it does, it will form a third, new compound. The third compound should contain both chiral centres: the one from the target compound and the one from the chiral resolving agent.
That changes everything. The compound contains two chiral centres. One of these is identical among all the molecules in the sample; that's the one from the pure chiral resolving agent. The other one is present in either of two configurations; that's the one from the enantiomeric target compound. That means we now have a mixture of diastereomers.
- Two enantiomers have the same physical properties. They cannot be separated easily by standard laboratory techniques.
- Two diastereomers have different physical properties. they can often be separated via standard laboratory techniques.
- If an additional chiral center can be incorporated into a pair of enantiomers so that they become diastereomers, they can be separated.
The classic example of this method of obtaining one isomer of a compound involves the formation of a diastereomeric salt. The salt has multiple chiral centers, and so diastereomers are possible.
If a racemic mixture of phenylsuccinic acid is mixed with a pure sample of (-)-proline (a naturally available amino acid), a proton transfer (or Bronsted acid-base) reaction occurs. Two protons are transferred from the phenylsuccinic acid to the proline. One proline has a position available for one extra proton, so the two protons end up on two different prolines. Because protons have +1 charge, each proline is cationic. The phenylsuccinic acid gave up two positives, so it is a dianion. Together, these three ions form a salt.
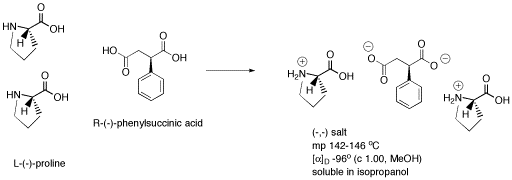
Figure SC14.3. Formation of a salt containing three chiral centers.
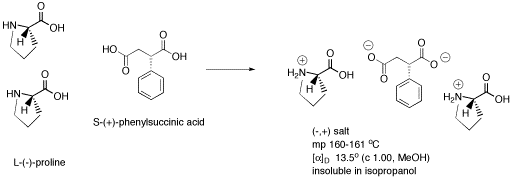
Figure SC14.4. Formation of a salt containing three chiral centers. This one is a diastereomer of the salt in the previous picture.
If a racemic mixture of phenylsuccinic acid is used, but pure (-)-proline is added, two possible diastereomers result. One chiral center, in the proline, is always the same. The other chiral center, in the phenylsuccinic acid, can be in two different configurations. As a result, cations and anions may pack differently together in each case, so different melting points and solubilities result. One salt precipitates or forms a solid from the solution, but the other stays dissolved. The two diastereomers can be separated by filtration.
Problem SC14.1.
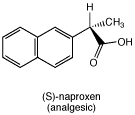
Suppose you have a pure sample of L-phenylalanine. Write equations for reactions, using structures, that show how you could use it to obtain a sample of (S)-naproxen, an analgesic, from a racemic mixture of naproxen.