SC7. The polarimetry experiment
In measuring optical rotation, plane-polarized light travels down a long tube containing the sample. If it is a liquid, the sample may be placed in the tube as a pure liquid (its is sometimes called a neat sample). Usually, the sample is dissolved in a solvent and the resulting solution is placed in the tube.
There are important factors affecting the outcome of the experiment.
-
Optical rotation depends on the number of molecules encountered by the light during the experiment.
-
Two factors can be controlled in the experiment and must be accounted for when comparing an experimental result to a reported value.
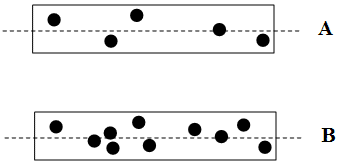
FigureSC7.1. The effect of concentration on optical rotation.
-
The more concentrated the sample (the more molecules per unit volume), the more molecules will be encountered.
-
Concentrated solutions and neat samples will have higher optical rotations than dilute solutions.
-
The value of the optical rotation must be corrected for concentration.
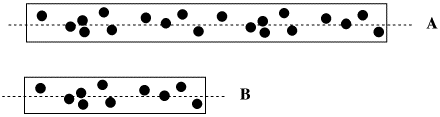
FigureSC7.2. The effect of path length on optical rotation.
- The longer the path of light through a solution of molecules, the more molecules will be encountered by the light, and the greater the optical rotation.
- The value of the optical rotation must be corrected for the length of the cell used to hold the sample.
In summary:
[a] = a / (c x l)
- a is the measured optical rotation.
- c is the sample concentration in grams per deciliter (1 dL = 10 mL).
- That is, c = m / V (m = mass in g, V = volume in dL).
- l is the cell length in decimeters (1 dm = 10 cm = 100 mm)
- The square brackets mean the optical rotation has been corrected for these variables.
Problem SC7.1. A pure sample of the naturally-occurring, chiral compound A (0.250 g) is dissolved in acetone (2.0 mL) and the solution is placed in a 0.5 dm cell. Three polarimetry readings are recorded with the sample: 0.775o, 0.806o, 0.682o.
a) What is [a]?
b) What would be the [a] value of the opposite enantiomer?
Problem SC7.2. A pure sample of the (+) enantiomer of compound B shows [a] = 32o. What would be the observed a if a solution of the sample was made by dissolving 0.150 g in 1.0 mL of dichloromethane and was then placed in a 0.5 dm cell?
