CO15. Proton Transfer in Carbonyl Addition
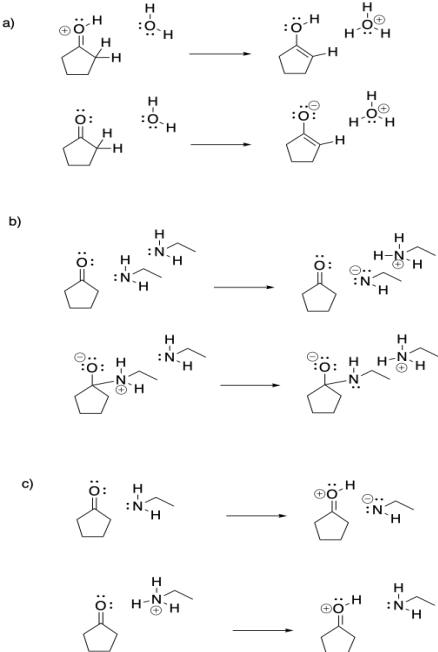
Proton transfer is rapid, especially if it is transferred from a very acidic position. For example, a proton can easily be transferred from a positively charged oxygen atom to a neutral oxygen (resulting in a new, neutral oxygen and a new, positive oxygen). These species would be in equilibrium with each other.
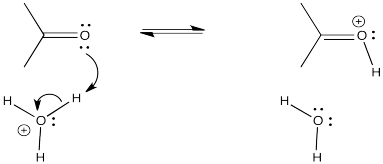
Figure CO15.1. Proton transfer to a carbonyl.
- Proton transfer is rapid.
- Protons can be transferred from more acidic to less acidic position.
- Protons can be transferred from one acidic position to another of similar acidity, although the equilibrium may not be favoured.
It would not be as easy to transfer a proton from a neutral oxygen to another neutral oxygen. Sometimes, a neutral oxygen can transfer a proton to a negatively charged one, but the equilibrium will depend on the relative pKa values of the two species. In the case below, the tert-butoxide is a less stable anion than the hydroxide because the tert-butoxide is larger and requires more organization of solvent molecules around it.
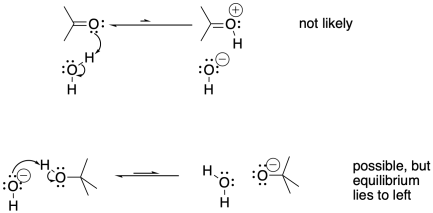
Figure CO15.2. Proton transfers are not always favoured.
It is tempting to think that a proton could be transferred directly from a cationic position to an anionic position in the same molecule. That might not occur, however. In terms of conformational analysis, the two positions might not be able to twist around and reach each other. The usual rule applies: two atoms may need to be greater than five atoms away from each other along a chain before they can reach around and make contact.
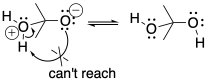
Figure CO15.3. Intramolecular proton transfers are not always favoured.
If the solvent has a lone pair, it may pick up the proton from the acidic position and drop it off on the basic position. These events are made easy by the fact that the reacting molecules are usually surrounded by many solvent molecules.
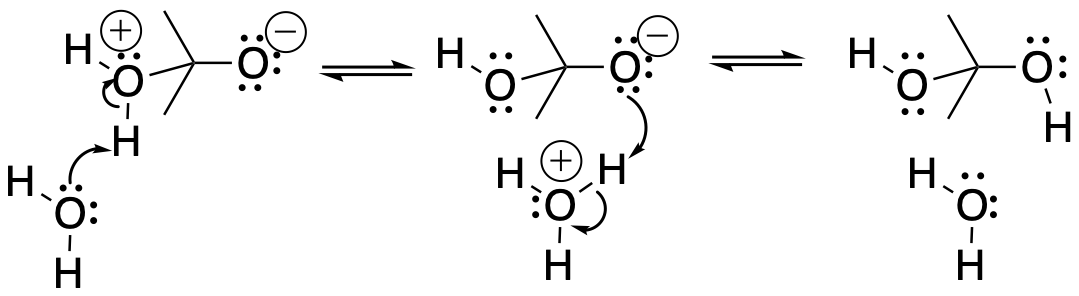
Figure CO15.4. Solvent-assisted proton transfer.
- Solvent can often act as a proton shuttle.
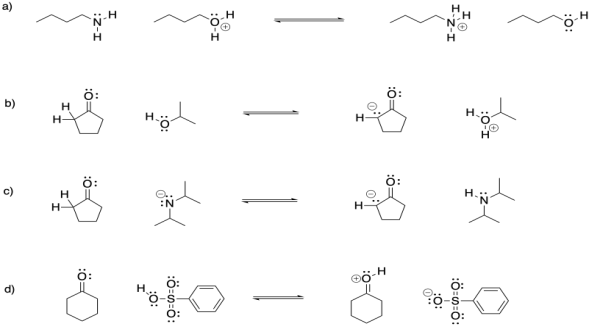
Problem CO15.1.
Circle the right side or the left side of each reaction to indicate the direction of equilibrium.
Problem CO15.2.
Circle the reaction the more favoured reaction in each of the following pairs.