CO13. Activation of Carbonyls
A secondary theme in carbonyl chemistry centers on the role played by the oxygen lone pairs. A compound with lone pairs can act as a Lewis base. Can carbonyl compounds also act as Lewis bases? The answer is yes, although it is most important to think about carbonyls primarily as Lewis acids.
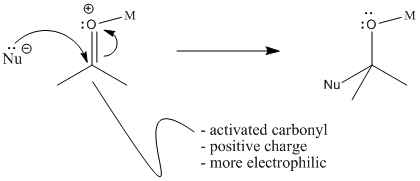
One of the reasons the basicity of the lone pair matters is because of carbonyl activation. If a carbonyl donates a lone pair to a Lewis acid, forming a bond, the carbonyl gets a formal positive charge. If the carbonyl has a formal positive charge, it attracts electrons more strongly. In that case, nucleophiles react more easily with the carbonyl. The carbonyl is said to be activated.
Figure CO13.1. Activation of a carbonyl via donation to a proton.
A carbonyl can be activated by the addition of a proton donor, such as HCl or other common acids.
- An activated carbonyl has a positive charge.
- Carbonyls become activated by donating a lone pair to a Lewis acid (also called an electrophile).
- Once activated, carbonyls become more reactive.
- Activated carbonyls attract nucleophiles more strongly.
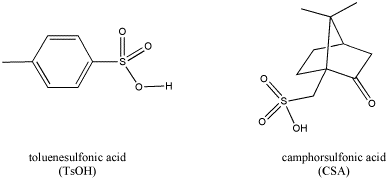
Most common mineral acids are used as aqueous solutions (the familiar HCl, H2SO4, HNO3, H3PO4 and so on). The acid is only found in the presence of water. Many of them are actually hydrates; if you take sulfuric acid, H2SO4, and set it to boil on a hotplate, eventually it reverts back to sulfur trioxide, SO3, as the water boils away, and a fog appears above the beaker. Sometimes, in a laboratory reaction, it isn't helpful to have all that water around (the reasons will become clear later). Other, organic acids are sometimes used instead, such as camphorsulfonic acid or toluenesulfonic acid; these are both solids that are easy to weight out and add to a reaction, and they don't add a bunch of water to the reaction.
Figure CO13.2. Some protic acids useful in activating carbonyls.
Carbonyls are also activated by more general Lewis acids. Often, metal chloride salts are used. These may include main group metals, such as aluminum, bismuth or indium, or transition metals such as scandium, titanium or iron.
Figure CO13.3. Activation of a carbonyl by a metal ion.
Once the carbonyl is activated, nucleophiles are more strongly attracted to the carbon. The carbon was already partially positive, but with a full positive charge on the molecule, electrons are attracted much more strongly.
It is tempting to donate electrons from a nucleophile to the positive oxygen. However, the oxygen already has three bonds and an octet. Remember, donating a lone pair from a nucleophile means the lone pair is becoming a bond between the nucleophile and the electrophile. Giving a pair of electrons directly to the oxygen would give it four bonds and more than an octet-- it would have 10 electrons. Instead, donation to the neighbouring carbon allows the C=O pi bond to move to the oxygen and become a lone pair. The positive charge on the oxygen disappears.
-
The nucleophile donates to the activated carbonyl carbon
-
That event lets the pi bond become a lone pair on oxygen
Figure CO13.4. Donation of nucleophile to an activated carbonyl.
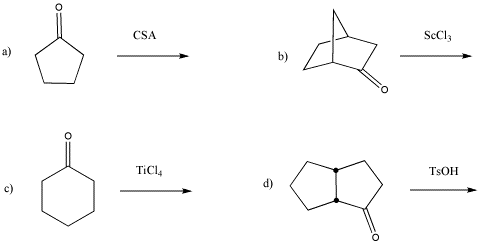
Problem CO13.1.
Show, with arrows, the activation of the following carbonyls.
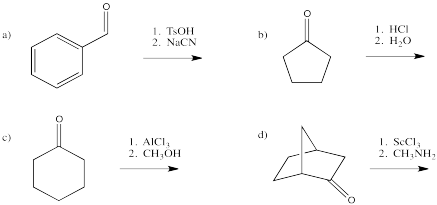
Problem CO13.2.
Show, with arrows, the activation of the following carbonyls, followed by donation from the nucleophile.
Problem CO13.3.
Part a. Use curved arrows to denote electron flow in the following mechanism for the Mukaiyama Aldol Addition.
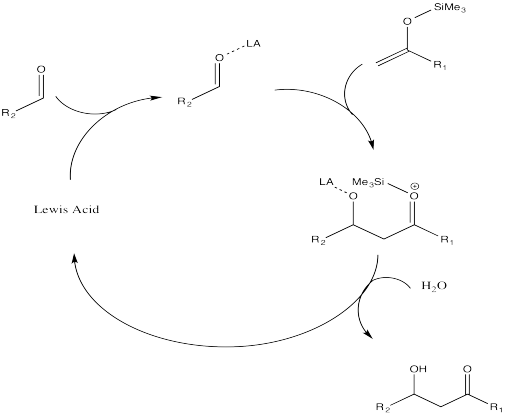
Part b. Using these starting molecules in Mukaiyama Aldol Addition, predict the product formed.
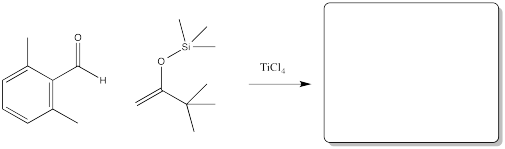
Problem CO13.4.
Provide Lewis-Kekule structures for the following commonly used acids.
a) hydrochloric acid, HCl b) hydrofluoric acid, HF c) hydrobromic acid, HBr
d) nitric acid, HNO3 e) perchloric acid, HClO4 f) phosphoric acid, H3PO4
g) formic acid, HCO2H h) acetic acid, CH3CO2H i) benzoic acid, C6H5CO2H
j) sulfuric acid, H2SO4 k) toluenesulfonicacid, CH3C6H4SO3H l) methanesulfonic acid, CH3SO3H