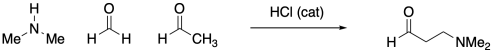CO16b. Neutral Nucleophiles: Putting the Steps Together
The addition of neutral nucleophiles, such as alcohols and amines, to carbonyl compounds, such as aldehydes and ketones, generally replaces the carbonyl oxygen altogether, replacing it with alkoxy or amino groups. The mechanisms for these reactions can look a little daunting. There are a lot of steps. If you stop and take a closer look, the steps are a little repetitive. They aren't that bad if you take things one step at a time.
Take the conversion of an aldehyde to an acetal. Two alcohol groups replace the carbonyl oxygen, but it doesn't all happen at once.
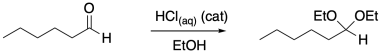
Figure CO16b.1. Acetal formation.
First of all, we have an acid-catalyzed reaction here. Usually, that means the carbonyl requires activation to become a better electrophile. Activation allows the normally unreactive neutral nucleophile to undergo addition with the carbonyl. In most cases, acid catalysis also means we will deal with a number of cationic intermediates throughout the mechanism. The hydroxy group will also need to be activated before it can leave, and it will be pushed off by π-donation, generating a C=O(+), much like an activated carbonyl.
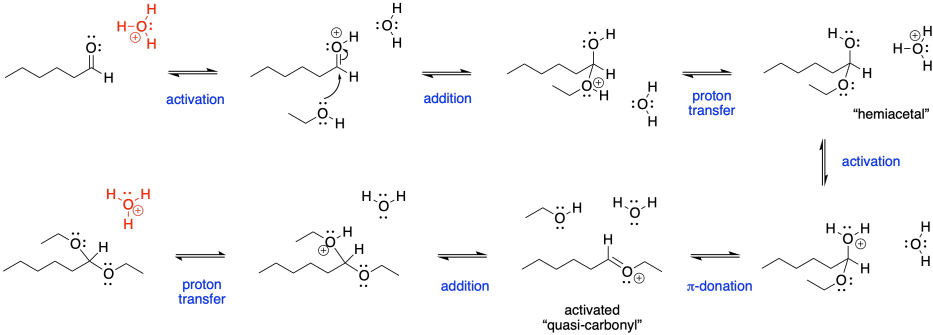
Figure CO16b.2. All the steps in acetal formation.
Note that the catalytic species, in this case shown as a hydronium ion in red, is regenerated at the end of the reaction. It is ready to start a new sequence of steps with the next aldehyde.
Problem CO16b.1.
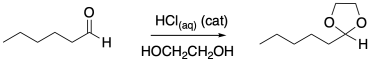
Diols can also react with carbonyl compounds to give cyclic acetals. Provide a mechanism for this reaction.
Amines typically do not add twice to carbonyls the way alcohols do. Instead, the single nitrogen that replaces the carbonyl forms a double bond to the carbon and loses a proton to become an imine.
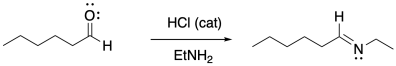
Figure CO16b.3. Imine formation.
Despite the very different product compared to alcohol addition, the mechanism is actually quite similar. Once again, there is acid catalysis in this case, although the proton is likely delivered by an amine proton carrier. The key step is loss of water via π-donation from the nucleophilic atom. Once again, as soon as that atom (a nitrogen in this case) has lost its original proton, it has a new lone pair to donate to the carbon, forming a double bond.
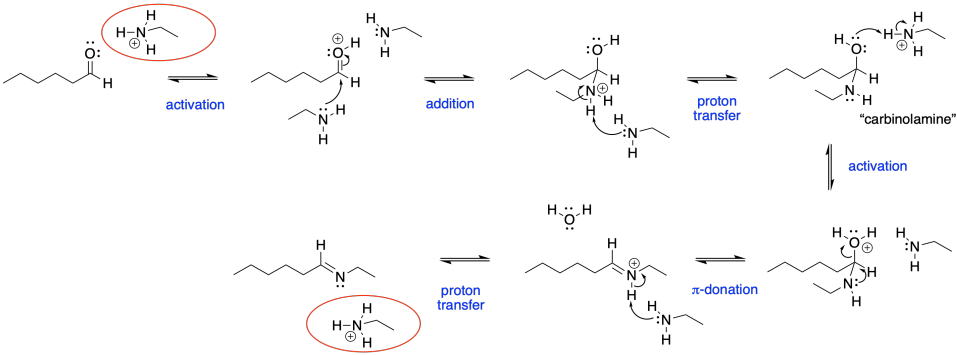
Figure CO16b.4. All the steps in imine formation.
Problem CO16b.2.
Amines can also react with carbonyl compounds to give enamines. Provide a mechanism for this reaction.
Problem CO16b.3.
Imines are easily converted back into carbonyl compounds through the addition of water and a catalytic amount of acid.
a) Provide a mechanism for this reaction.
b) Describe each step as "proton transfer", "π-donation", etc.
c) Circle the catalytic species where it appears at the beginning and end of the mechanism.
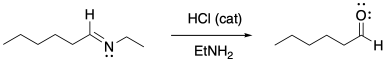
Problem CO16b.4.
The Mannich reaction is a historically important route for the preparation of alkaloids. The reaction involves an iminium ion electrophile and an enol nucleophile.
Provide a mechanism for the reaction.