CO23. Conjugate Addition & Elimination in Aromatics
In conjugate addition, a carbonyl group turns a neighbouring alkene into an electrophilic site. An enone, such as the one below, has two electrophilic positions. The carbonyl carbon is electrophilic just like always. However, there is also an alkene carbon that is electrophilic, too. That's unusual because alkenes are not sites of partial positive charge, in general. They are electron-rich. In fact, alkenes are usually nucleophilic, not electrophilic. It is only under the influence of the carbonyl that the conjugated alkene become an electrophile.
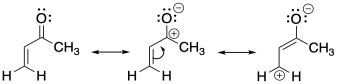
Figure CO23.1. Electrophilic positions in an enone.
A similar situation happens when pi-acceptors such as nitro groups are attached to aromatic rings. Aromatic rings are electron-rich. A benzene, for example, contains three pi bonds, not just one. It seems even more electron-rich than an alkene. In general, aromatic rings behave as nucleophiles because they are electron-rich. Adding a nitro (NO2) group to the benzene can change things. A nitro group is electron-withdrawing enough that it can attract a nucleophile to the otherwise electron-rich benzene. If there is a halogen placed in the right place on the benzene, the nucleophile can replace it in a substitution reaction.
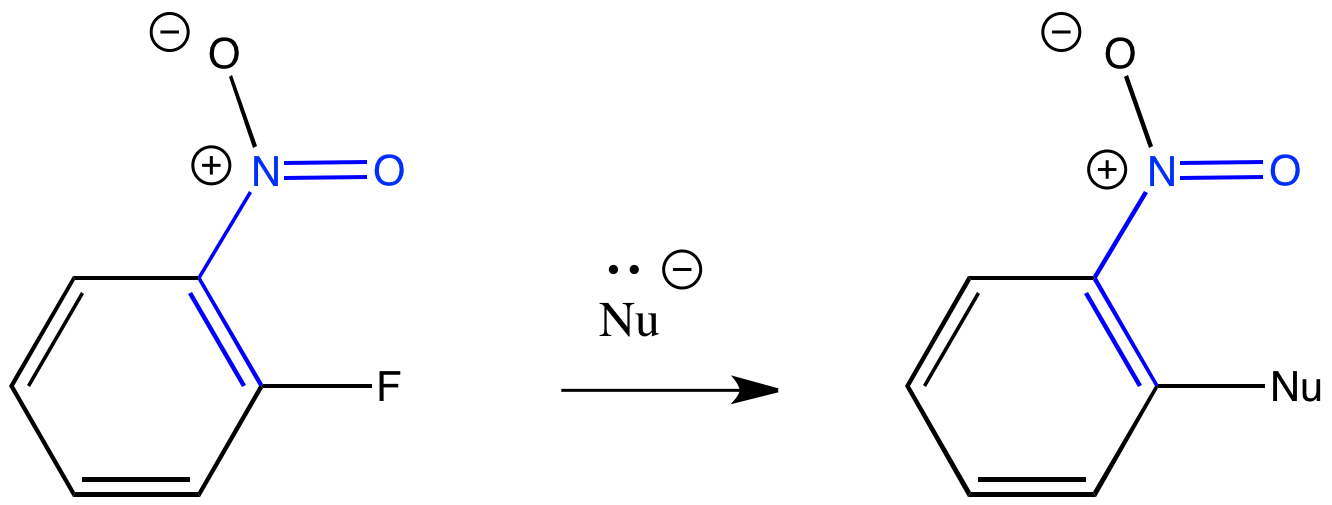
Figure CO23.2. A conjugate addition-elimination of an aromatic or "nucleophilic aromatic substitution".
One sign that this is different from a regular nucleophilic substitution reaction (if you are familiar with that reaction) is the nature of the leaving group. Normally, fluoride isn't a good leaving group for this kind of substitution. Here, the fluorine works really well because it is so electronegative. It helps turn something that should normally be a nucleophile into an electrophile. It is much easier to replace a fluorine in these reactions than it is to replace a chlorine, bromine, or iodine. The reactivity of these halogens drops as their electronegativity decreases.
The most common nucleophiles in these reactions include hydroxide and alkoxides such as NaOCH3. Sometimes other nucleophiles such as ammonia or amines are used.
When the nucleophile adds to the double bond of the benzene, we get an anionic intermediate. Notice thet there are two negative charges and one positive charge, for an overall negative charge. There are several resonance structures for this intermediate; the one drawn here places the negative charge on the electronegative oxygen of the nitro group. That anionic intermediate is the reason this reaction requires an electron-withdrawing group such as a nitro group. It's also the reason why an electronegative fluorine helps the reaction.
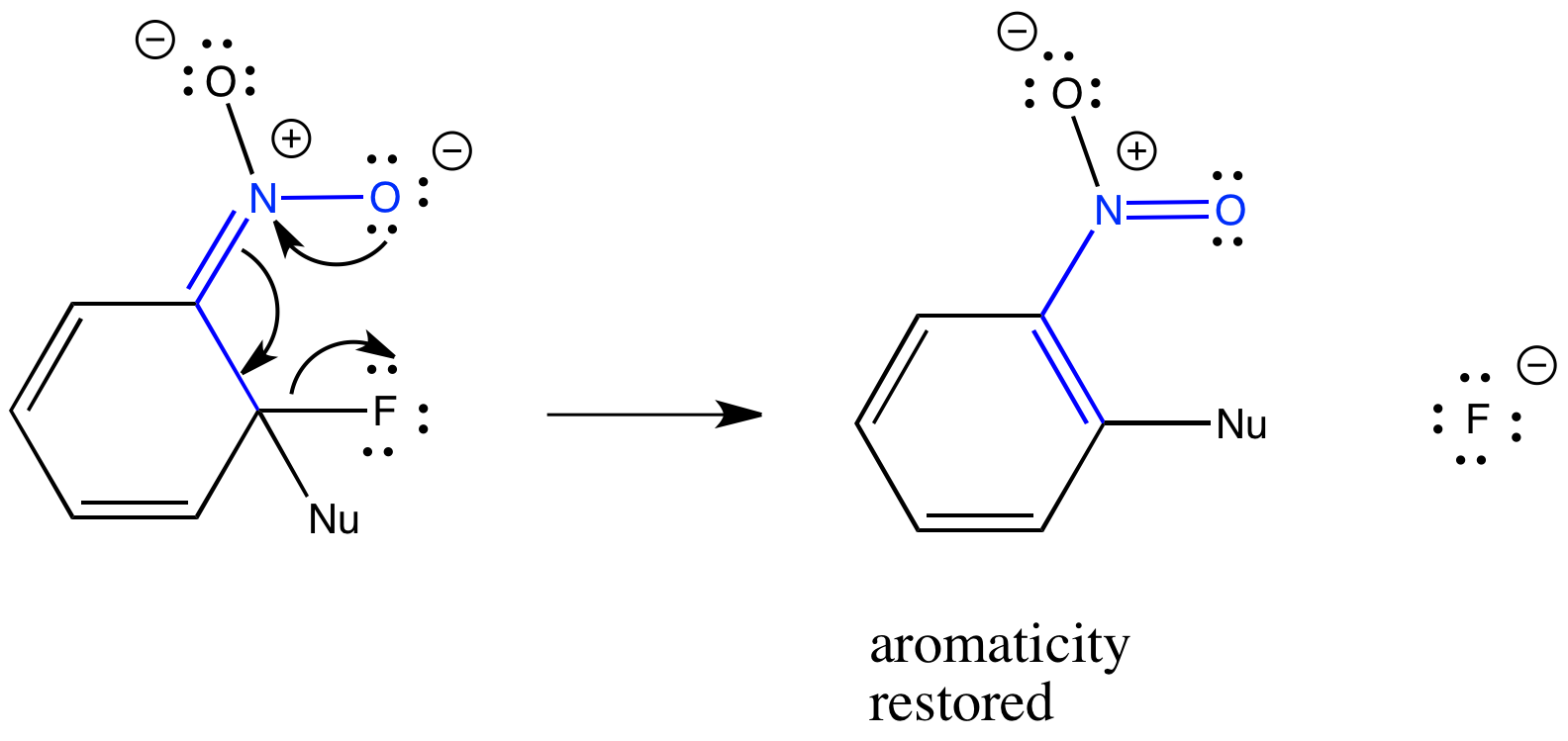
Figure CO23.3. The key step in addition-elimination of an aromatic.
The key step in the mechanism is the loss of the halide ion, which allows the aromaticity to be restored. It's a two-step process: first, the nucleophile adds to make an anionic intermedate; second, the leaving group leaves and takes the negative charge with it. Sometimes, people refer to this process as an addition-elimination mechanism, reflecting those two steps. Other people refer to it as a nucleophilic substitution at an aromatic (SNAr is an abbreviated term that means the same thing).
Problem CO20.2.
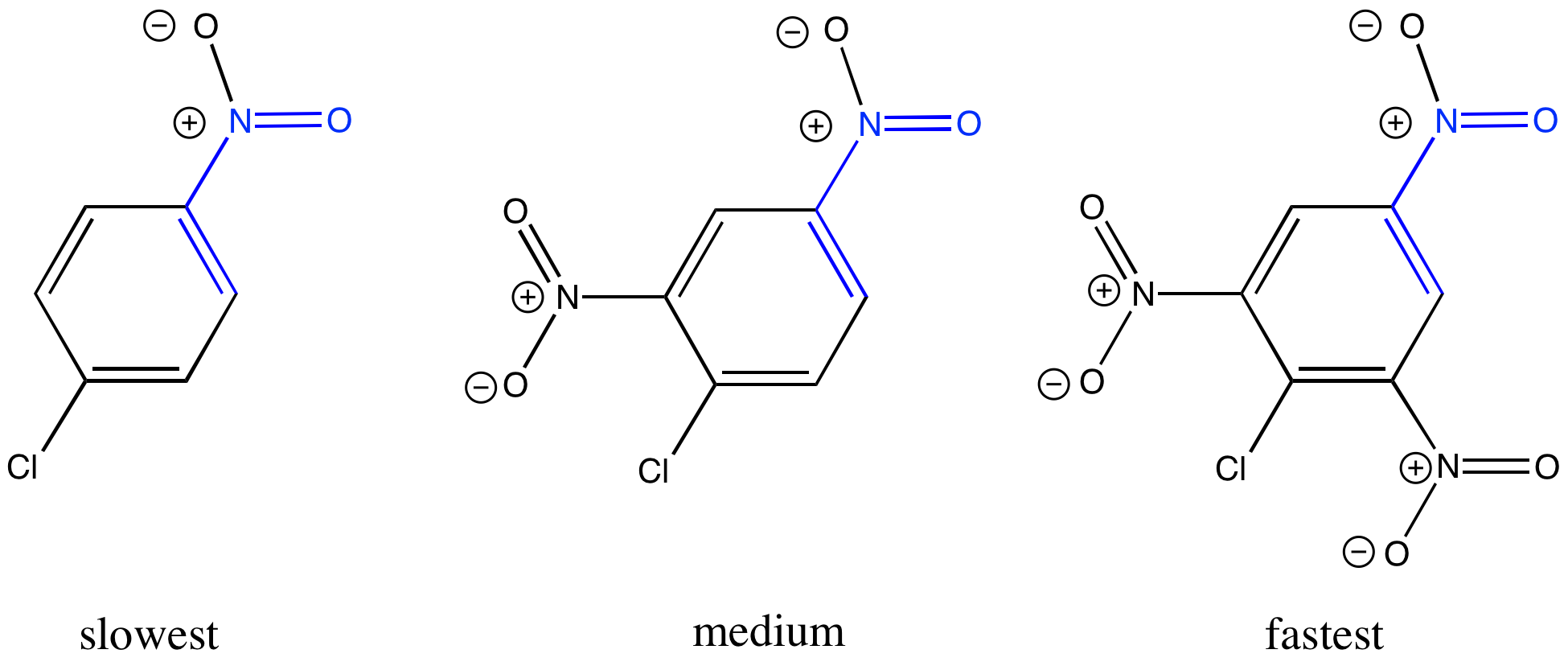
The location of the halogen and the electron-withdrawing group matters. Explain why the reaction occurs if the groups are in the 1 and 2 positions (ortho to each other) or the 1 and 4 positions (para to each other), but not if they are in the 1 and 3 positions (meta to each other).
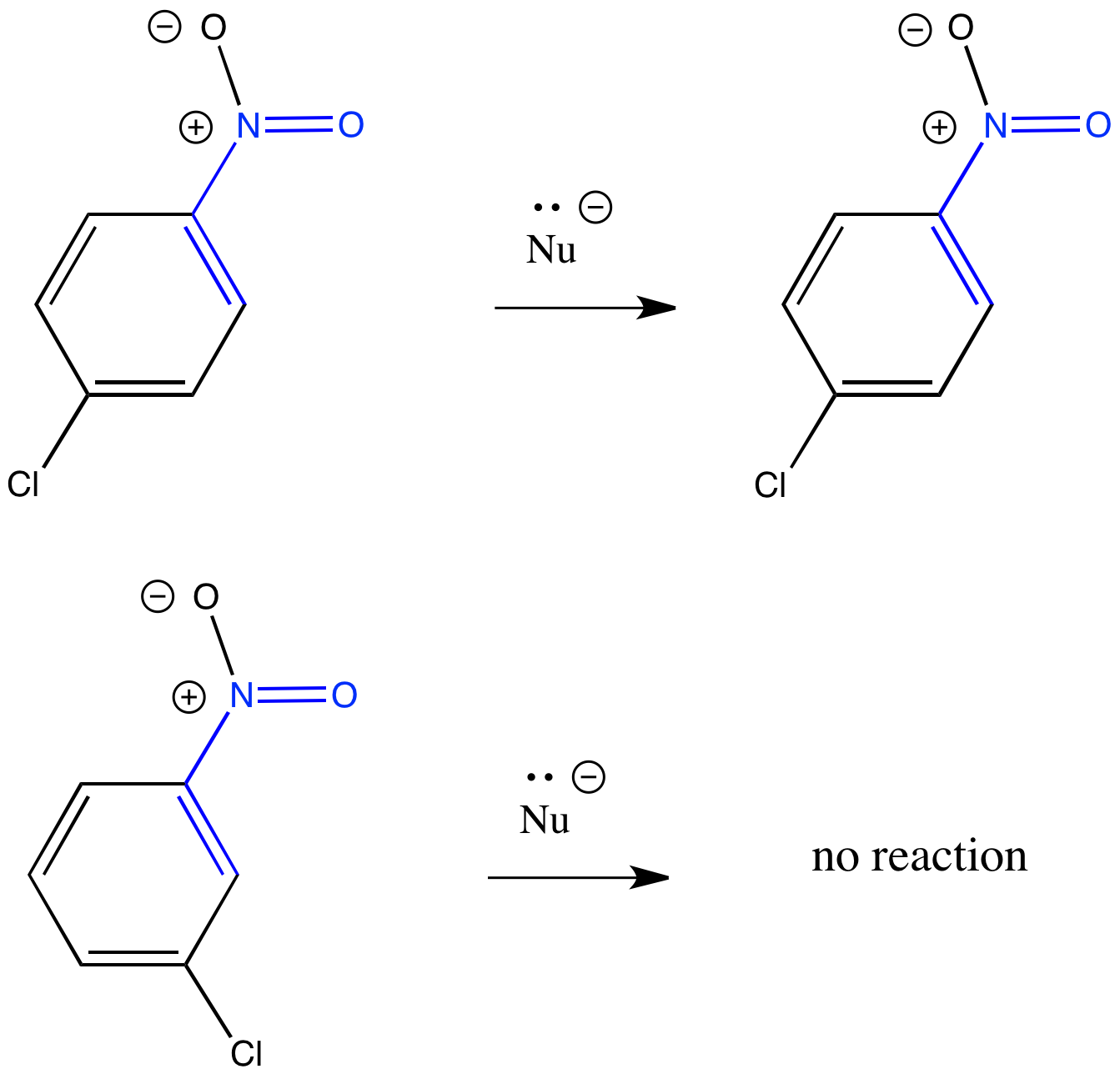
Figure CO23.4. The crucial placement of electron withdrawing groups.
Problem CO20.3.
Explain why the reaction is faster if additional electron-withdrawing groups are present.