Reactivity in Chemistry
Carbonyl Addition
CO1. Carbonyls are Found
in Biological
Molecules
Organic compounds are a broad
class of compounds that got their name because they were originally found in
living, or organic, matter. Physicians during the Renaissance and
afterward strove to develop new medicines by extracting "active principles" from
plants that were known to have medicinal properties. The same practice
remains the most common method by which pharmaceutical companies develop
medicines today.
Carbonyls are organic compounds
that contain C=O bonds; that is, they contain double bonds between carbon and
oxygen. (The word is pronounced car-bow-KNEEL.) This group of compounds is
probably the most important single class of organic molecules. They can be
divided into two classes. There are simple carbonyls, in which the carbon
of the C=O bond is attached to other carbons, or possibly to hydrogens.
There are also carboxylic acid derivatives, or carboxyloids, in which the
carbony carbon is attached to a "heteroatom": an atom other than carbon or
hydrogen, such as oxygen, nitrogen, sulfur, or a halogen.
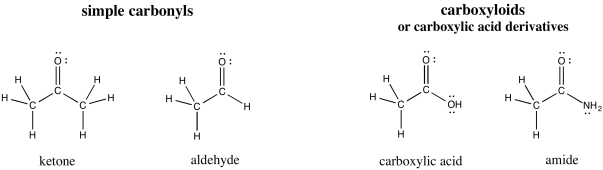
Figure CO1.1. Some
carbonyl compounds.
Carbonyl compounds are very
common in biological chemistry. Classes of compounds that contain the C=O bond
include amides (found in proteins & peptides, used as signaling molecules and to
help catalyze and guide reactions), aldehydes and ketones (found in
carbohydrates, which play structural roles in cellulose, starch and DNA, for
example) and esters (found in fats that form cell membranes, among other
things). Understanding the reactivity of these bonds will help you to learn
about many biological processes, as well as other transformations that are
important in human society.
Common clusters of atoms, such as
the H-C=O group in an aldehyde or the HO-C=O group in a carboxylic acid, are
called "functional groups".
The amino acids are some of the
fundamental building blocks of life. Fundamentally, they are carbonyl
compounds.
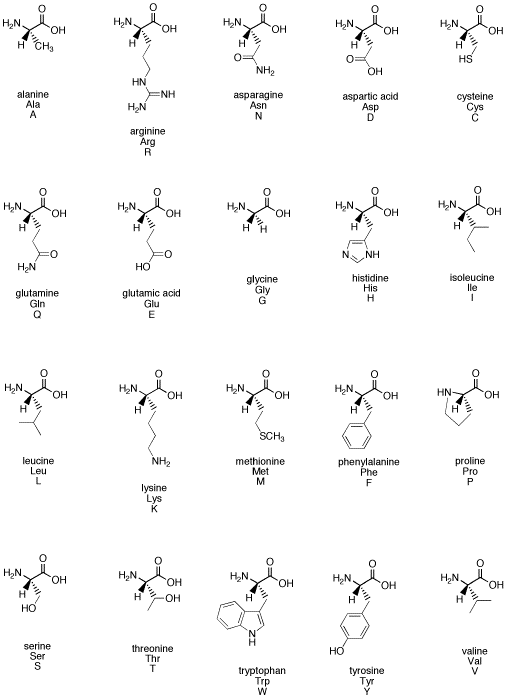
Figure CO1.2. An
alphabet of amino acids illustrates how common carbonyls are in biological
chemistry.
Problem CO1.1.
What functional groups
contain carbonyls in the family of amino acids illustrated above?
Amino acids are connected
together to form peptides. Peptides are "polymers", which means they
are very large molecules made up of small, repeating units.
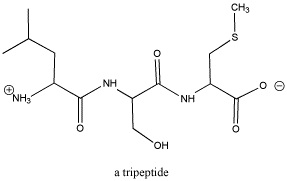
Figure CO1.3. A tripeptide, composed of three
amino acids. See if you can identify the three separate sub-units.
Carbohydrates are another important class of
biomolecules. They also contain carbonyl groups. Like amino acids,
there are many variations of carbohydrates, and they are sometimes found bound
together to form polymers. Of course, carbohydrates are important in
energy storage. In plants, they also provide structural strength, helping
to form cell walls.
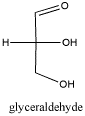
Figure CO1.4. A simple carbohydrate,
glyceraldehyde.
Problem CO1.2.
Identify which group is
contained in each of the following carbohydrates: an aldehyde or a ketone?
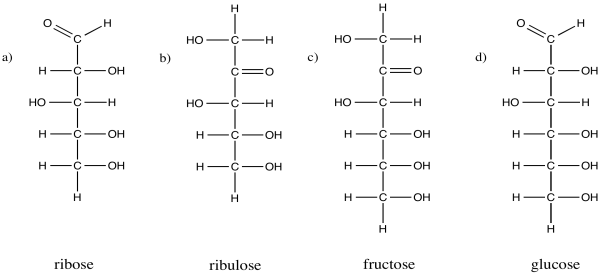
Fatty acids, triglycerides and phospholipids are a
third class of biomolecules that contain carbonyls. These compounds are
also used in energy storage. In addition, phopholipids form a major part
of cell membranes, so these compounds also play an important structural role in
biology.
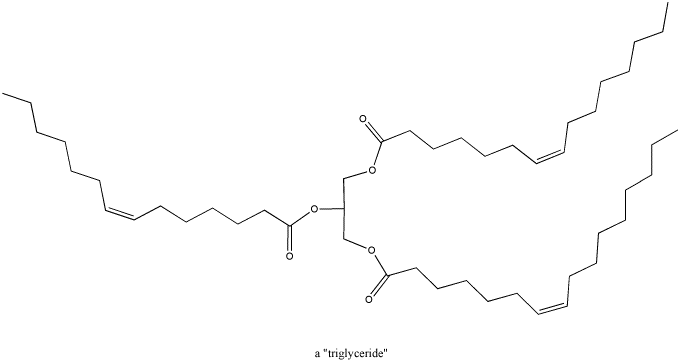
Figure CO1.5. A triglyceride.
Problem CO1.3.
Locate the carbonyls in the
following biological molecules and the functional groups that contain
carbonyls in each case.
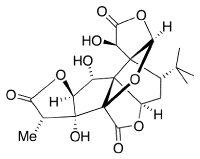
Ginkgolides are biologically
active terpenoids from Ginkgo trees. They are thought to have
medicinal properties.
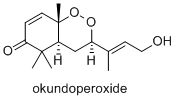
Okundoperoxide is isolated
from a type of sedge in Cameroon. It has modest anti-malarial
properties.
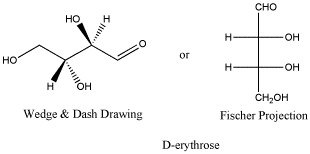
D-erythrose is a typical
carbohydrate.
Problem CO1.4.
Frequently, the carbonyls in
carbohydrates are "masked", as in deoxyribose (below).
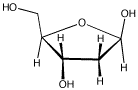
By moving one proton from one
position to another, and then breaking a single C-O bond, discover
where the carbonyl is hiding.
This chapter will focus on the reactivity of simple
carbonyl compounds: the aldehydes and ketones. Later, we will look at the
reactivity of carboxyloids. Together, these two different sets of
reactions can be used to explain a great deal of molecular processes in biology.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's
University (retired) with other authors as noted on individual pages. It is freely
available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation
under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this
material are those of the author(s) and do not necessarily reflect the views of
the National Science Foundation.
Navigation:
Back to Carbonyl Addition Index
Back to Reactivity
Back to Web Materials on Structure & Reactivity in Chemistry










