CO2. Carbonyls are Electrophiles
The carbonyl bond is very polar. There is a partial positive charge on the carbon and a partial negative charge on the oxygen, because oxygen is more electronegative than carbon. This charge separation is intensified because of the double bond between the carbon and oxygen. Rather than just pulling one pair of bonding electrons towards itself, the oxygen pulls two pairs of electrons towards itself.
- The C=O bond is very polar.
- The carbonyl carbon is very positive.
Sometimes, a resonance structure is drawn to emphasize the charge separation in the carbonyl. The structure has only one bond between the carbon and oxygen. In this structure, oxygen has an octet but carbon does not. This is not really a good Lewis structure, because the other resonance structure satisfies octets on all the atoms. However, this Lewis structure emphasizes the polarity of the bond and is sometimes drawn to reinforce that idea.
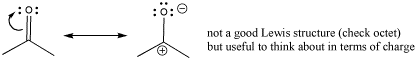
Figure CO2.1. Thinking about charge distribution in a carbonyl group.
Because of the positive charge on the carbonyl carbon, the most important theme in carbonyl chemistry is reaction of the carbonyl as a Lewis acid. Reactions of carbonyls almost always involve addition of an electron donor to the carbonyl carbon.
- Electrophile is another term for Lewis acid.
- Lewis acids attract electrons.
- Lewis acids have a positive charge on an atom, a partial positive charge on an atom, or an atom lacking an octet.
- Carbonyl compounds are good electrophiles.
The electrophilicity of carbonyls is very important in their reactivity. The goal of this chapter is to develop an understanding of how carbonyls react. We will learn about a few key factors that will be used in different combinations under different circumstances. Eventually, you will build an understanding that will allow you to follow both biological reactions and modern synthetic reactions.
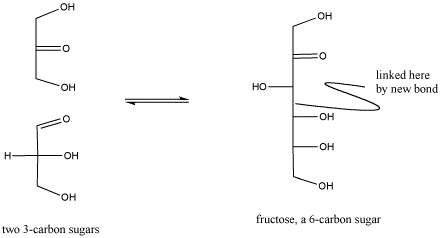
Figure CO2.2. Reactivity in carbonyl compounds. The carbonyl in the lower sugar on the left has reacted with the neighbouring molecule.
It is important to realize that biological reactions, such as carbohydrate synthesis, are very complex and can involve many, many steps. For example, the carbohydrate synthesis shown above involves additional acid-base steps as well as a reaction of a carbonyl. The additional acid base steps may involve proton donors and acceptors as well as more general Lewis acids.
Problem CO2.1.
a) Explain why the carbon in a C=O unit is very electrophilic, but the carbon in a C-O unit is much less so.
b) Propose other carbon-heteroatom bonds that may make the carbon electrophilic (heteroatom means not carbon or hydrogen).
Problem CO2.2.
Provide line structures for the following
compounds.