CO19. Biological Reduction
Addition to a carbonyl by a semi-anionic hydride, such as NaBH4, results in conversion of the carbonyl compound to an alcohol. The hydride from the BH4- anion acts as a nucleophile, adding H- to the carbonyl carbon. A proton source can then protonate the oxygen of the resulting alkoxide ion, forming an alcohol.
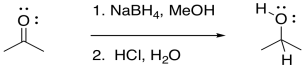
Figure CO19.1. Addition of hydride to a carbonyl by NaBH4.
Formally, that process is referred to as a reduction. Reduction generally means a reaction in which electrons are added to a compound; the compound that gains electrons is said to be reduced. Because hydride can be thought of as a proton plus two electrons, we can think of conversion of a ketone or an aldehyde to an alcohol as a two-electron reduction. An aldehyde plus two electrons and two protons becomes an alcohol.
Aldehydes, ketones and alcohols are very common features in biological molecules. Converting between these compounds is a frequent event in many biological pathways. However, semi-anionic compounds like sodium borohydride don't exist in the cell. Instead, a number of biological hydride donors play a similar role.
NADH is a common biological reducing agent. NADH is an acronym for nicotinamide adenine dinucleotide hydride. Insetad of an anionic donor that provides a hydride to a carbonyl, NADH is actually a neutral donor. It supplies a hydride to the carbonyl under very specific circumstances. In doing so, it forms a cation, NAD+. However, NAD+ is stabilized by the fact that its nicotinamide ring is aromatic; it was not aromatic in NADH.
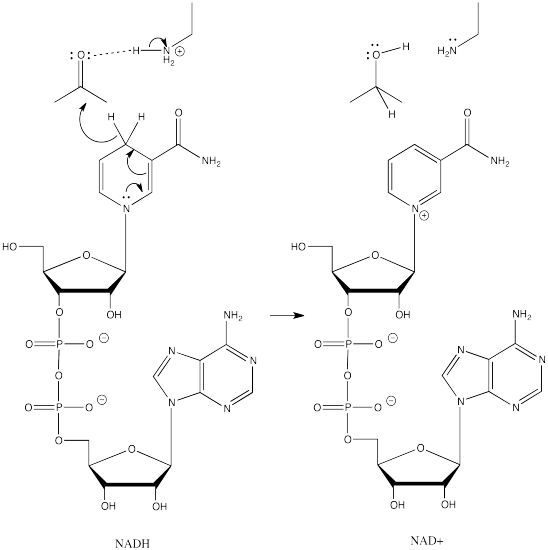
Figure CO19.2. Addition of hydride to a carbonyl by NADH.
So, in biological settings, we see NADH as a hydride donor instead of NaBH4 or LiAlH4. Instead of forming an alkoxide ion intermediate, RO-, that needs to be preotonated, NADH always works in sync with an amino acid side chain that will provide a proton as the NADH donates the hydride.
- NADH is an unusual source of nucleophilic hydride, H-.
- Hydride donation is boosted by pi donation, leading to an aromatic structure.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carbonyl Addition Index
Back to Web Materials on Structure & Reactivity in Chemistry