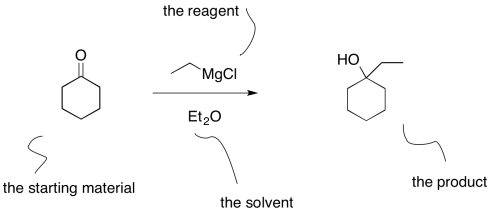
From the student's point of view, the information presented in an equation of a reaction can be confusing. The starting material and the product are linked by a straight reaction arrow. The starting material is the compound at the beginning of the reaction; the product is the compound at the end. The reagent is usually shown above the arrow. The reagent is the compound needed to turn the starting material into the product.
However, something else is often listed along with the reagent: the solvent. That can make students wonder: what does this thing do? Is it a second reaction I should be worrying about?

Figure CO10.1. One common way of writing an equation of reaction.
The solvent is just a liquid that is used to dissolve everything so that it can be mixed together better. Mixing things together better will allow the reaction to run more smoothly. Reagents will be able to find each other more quickly. Also, the solvent can act as a heat sink for the reaction, keeping the temperature a little more stable (this only works within limits, as solvents are sometimes flammable liquids and should not be overheated).
Students sometimes make the assumption that the reagent is written above the arrow and the solvent written below the arrow. That's a good observation, because reactions are often written that way, although there is no rule that says they have to be. However, there are exceptions in which that typical way of writing things is abandoned. Some reactions require lots of different reagents, additives, and promoters, or else there is a need to report the temperature or the pressure. In these cases, additional items are written below the arrow, just because there isn't enough room on top.
In other cases, a series of reactions are run. For example, in the above reaction it is assumed that there was an aqueous workup to neutralise the product. We might write that reaction out explicitly. In that case, the two different steps are numbered, so that we know that they were done one step at a time, rather than throwing everything in all at once.
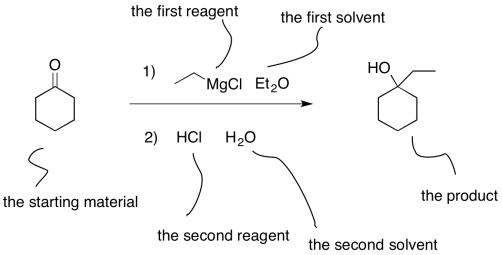
Figure CO10.2. One common way of writing an equation of a 2-step reaction.
Chemists often list the solvent in the reaction because the solvent is, practically speaking, tremendously important. Performing a reaction without solvent is a little like washing your hands without water. You could take a bar of soap and run it between your fingers, but not much will happen without the power of the water. The water dissolves up the soap (or at least suspends it in micelles), moves it around, gets it into contact with the dirt and carries it away.
In fact, water is literally the solvent in the physical process of washing. It can be a solvent in many chemical reactions as well. The solvent has many roles to play in a reaction. Foremost, it dissolves the reactants. In that state, the reactants are very mobile. Without the solvent, the reactants may be solids, or if liquids, they may be too thick for molecules to move around very quickly; they may be more like oils. Depending on the nature of the solvent, intermediates may be stabilised, allowing them to form more easily and aiding the course of the reaction. Solvents also act like baths, moderating heat flow into or out of the reaction as needed.
In the cartoon below, nothing happens when the two reagents are dumped together. When a solvent is added, the two reagents start to dissolve, and as they move around in the solution the two reagents encounter each other and start to react.
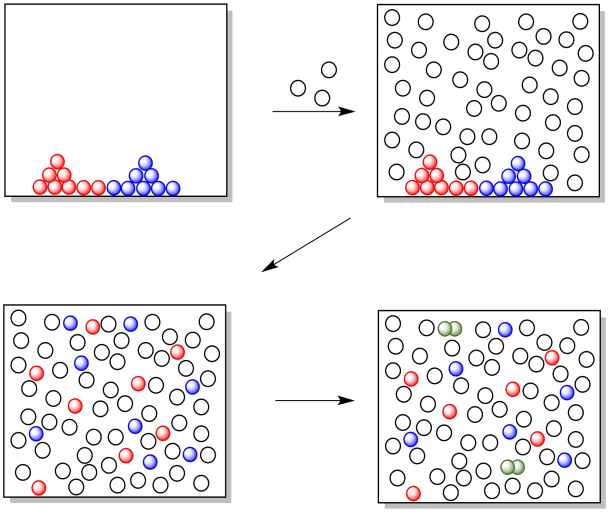
Figure CO10.3. The solvent mixes everything together.
At the beginning, you may not want to worry too much about the role of the solvent. However, you may still want to know what sorts of things are likely to be solvents, if only so that you can safely ignore them when trying to sort out how the reactant gets to the product.
The following table sums up a number of the most common solvents, displayed from most polar at the top to least polar at the bottom.
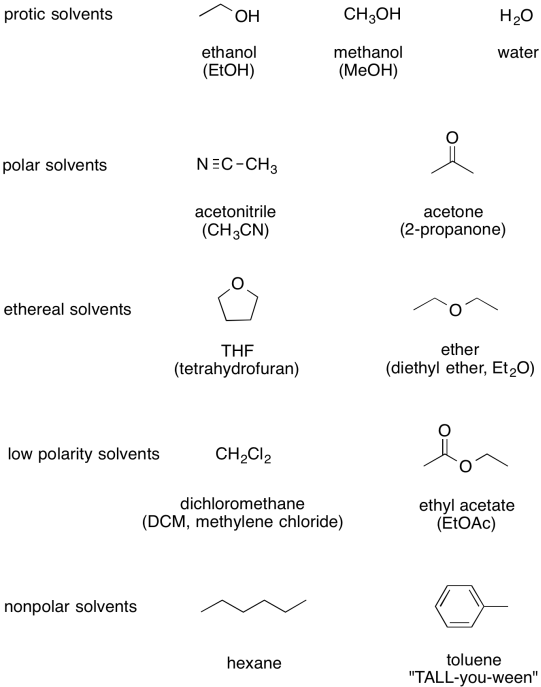
Figure CO10.4. Some common organic solvents.
Note that, just because something acts as a solvent in one reaction doesn't mean it must be one in another. For example, acetone is a pretty common solvent, but it also happens to be a ketone. It's likely to undergo carbonyl addition reactions if presented with good nucleophiles. For that reason, carbonyl addition reactions wouldn't be carried out with acetone, because the nucleophile would just react with the solvent instead of the intended electrophile.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carbonyl Addition Index
Back to Web Materials on Structure & Reactivity in Chemistry