CO17. Sugars: Pyranose and Furanose Forms
Carbohydrates are an important class of biological molecules. Although their best-known role is in energy storage in the form of glucose and starch, carbohydrates play a number of other roles. For example, they lend structural support in the backbone of DNA. They aid in homebody security and defense operations by forming molecular codes on the surfaces of cells that identify whether the cell is one of our own or an intruder. They carry specialized chemical reagents into enzymes where reactions essential to life are carried out. Some of them are sweet.
The term "sugar" is often applied to any simple carbohydrate. One strict definition of a carbohydrate is a polyhydroxylated aldehyde or ketone. In general, a sugar is a molecule that contains an aldehyde or a ketone, and every carbon other than the carbonyl carbon has a hydroxyl group attached to it.
This sort of structure presents lots of possibilities for reactions. In just one molecule, we have both an electrophile (the carbonyl) and a number of nucleophiles (the hydroxyls). Can sugars react with each other? Yes. Can a sugar react with itself? Of course.
In fact, if you have seen drawings of sugars before, you might not have noticed the carbonyl. That's because the carbonyl is usually "masked" as a hemiacetal. The hemiacetal forms when a hydroxyl group along the carbon chain reaches back and bonds to the electrophilic carbonyl carbon. As a result, five- and six-membered rings are very common in sugars. Five-membered rings are called "furanoses" and six-membered rings are called "pyranoses".
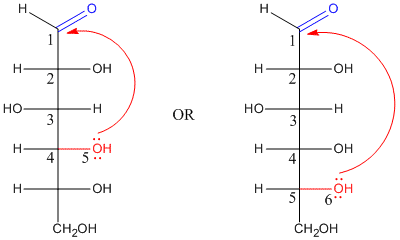
Figure CO17.1. Possible donations for hemiacetal formation in glucose.
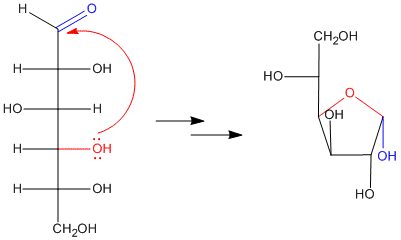
Figure CO17.2. Hemiacetal formation in glucose: making glucofuranose.
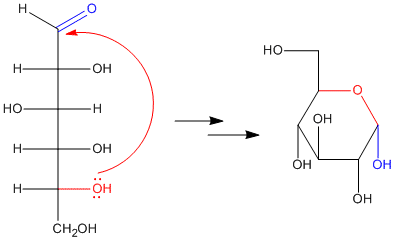
Figure CO17.3. Hemiacetal formation in glucose: making glucopyranose.
- Carbohydrates can make cyclic structures.
- These rings form through an intramolecular reaction: a carbonyl undergoes addition by an OH group farther down the chain.
- Most often six- or five-membered rings are formed this way.
The most common way of drawing these rings are in "Haworth projections". Haworth projections don't reflect the real shape of the ring. For example, in a six-membered ring, the atoms in the ring adopt a zig-zag, up-and-down pattern in order to optimize bond angles. The chair drawing shows that relationship, but in a Haworth projection, the ring is drawn as though it were flat. Also, substituents on the atoms in the ring can be found above the ring, below the ring, or sticking out around the edge of the ring. The chair drawing or "diamond lattice projection" shows these relationships pretty well. A Haworth projection doesn't try to do that. Instead, it tries to depict stereochemical relationships: whether two substituents are on the same face of the ring or opposite faces of the ring. In a diamond lattice projection, we have to keep track of whether a given substituent is in the upper or lower position at its particular site on the ring, and that requires careful attention. In a Haworth projection, substituents are simply drawn straight up or straight down. Because there are many chiral canters in sugars, and becuase two sugars can differ by just one chiral center, Haworth projections make it easier to tell different sugars apart.
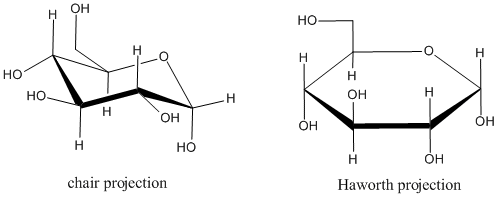
Figure CO17.4. Chair and Haworth drawings of α-(D)-glucopyranose.
When an open-chain sugar cyclizes by forming a hemiacetal, it forms a new stereocenter. Because the carbonyl carbon is trigonal planar, the hydroxyl group can approach it from either face. There is nothing to distinguish one face from the other, and so approach from either face is equally likely. That means that two different stereochemical configurations can form at the hemiacetal carbon: R and S.
In sugar chemistry, these two isomers are named a different way: alpha and beta. To distinguish these two designations, you need to look at the Haworth projection. In a Haworth projection, the lower edge of the ring is read as being nearer to you. The upper edge is read as being farther away. Remember, that's how we usually read a chair structure, too. However, in a Haworth projection, we have to orient the ring in a specific way. The hemiacetal carbon is always placed at the right edge of the drawing. In addition, we always keep the oxygen atom on the back edge of the ring (i.e. the upper edge of the drawing). That means the ring oxygen in a Haworth projection is always found in the upper or upper right part of the drawing, with the hemiacetal carbon directly beside it to the right.
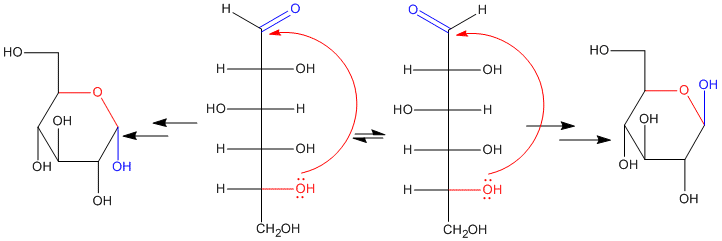
Figure CO17.5. Formation of anomers of glucopyranose.
If we have the Haworth projection, we can designate whether we have the alpha or beta from by seeing whether the hydroxy part of the hemiacetal points up or down. If it is down like the ants, we have an alpha isomer. If it is up like the butterflies, we have a beta isomer.
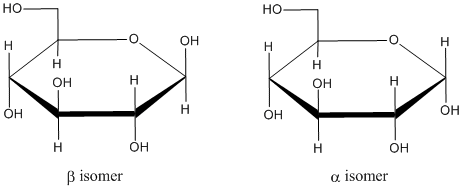
Figure CO17.2. The two anomers glucopyranose.
Because the hemiacetal carbon can adopt either of two configurations in a ring, it is given a special name. It is called the anomeric position.
Anomers are two diatereomers that form based on the stereochemical orientation of the OH group formed from the C=O. In the α-anomer, the new OH is on the opposite face of the ring from the group on the last chiral center around the ring (the CH2OH). In the β-anomer, the new OH is on the opposite face of the ring from the group on the last chiral center around the ring (the CH2OH). For (D)-glucopyranose, there is a mnemonic to keep the anomers straight: the OH is up with the butterflies (β) or down with the ants (α).
