Reactivity in Chemistry
Substitution at Carboxyloids
CX7b. Enolates: Decarboxylation
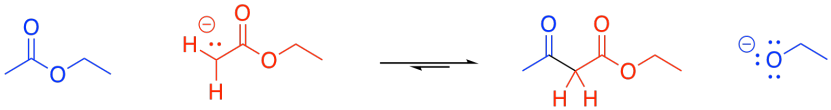
The formation of a beta-ketoester from two esters is called a "Claisen condensation".

Figure CX8.1. A Claisen condensation.
It is often followed by another important reaction: decarboxylation. If a beta-ketoester is treated with aqueous acid and heated, a couple of reactions take place. First, the ester portion of the molecule is converted into a carboxylic acid.
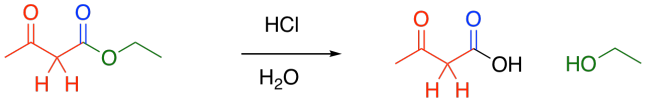
Figure CX8.2. Ester hydrolysis on a Claisen condensation product.
Second, the carboxylic acid is decarboxylated. Carbon dioxide is formed, and the organic molecule becomes a ketone. The carboxyl group is lost completely from the original molecule, and is converted into CO2.

Figure CX8.3. Decarboxylation of a β-keto acid.
Decarboxylation is related to the retro-aldol reaction; formally, it can be thought of as leading to an enolate leaving group. Decarboxylation most commonly occurs in beta-ketoacids, rather than in other carboxylic acids. Otherwise, that leaving group could not occur. The ease of decarboxylation in beta-ketoacids is related to the stability of the enolate anion.
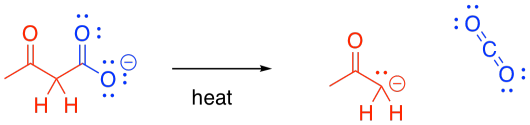
Figure CX8.4. A possible enolate leaving group in decarboxylation.
Under acidic conditions, of course, an enolate anion does not really occur. We don't normally get anionic intermediates under acidic conditions, only cationic or neutral ones. Instead, an enol is formed. However, enols are rapidly converted into the keto tautomers.

Figure CX8.5. The enol product of decarboxylation.
Decarboxylations are usually understood as pericyclic reactions, in which all of the bond-making and -breaking steps occur at once, generally through a cyclic transition state, with all of the electrons moving in a circle. In that case, the proton of the carboxylic acid would be transferred directly to the carbonyl oxygen to form the enol intermediate.

Figure CX8.6.The pericyclic mechanism of decarboxylation.
Pericyclic reactions are a little more sophisticated than the ones we are looking at now, so we will look more closely at them in a leter chapter.
Problem CX7b.1.
Draw a mechanism under acidic conditions for:
a) Conversion of ethyl-3-oxyhexanoate into 3-oxyhexanoic acid. (Oxy is a prefix meaning a ketone or aldehyde is foundalong the chain).
b) Decarboxylation of the resulting 3-oxyhexanoic acid.
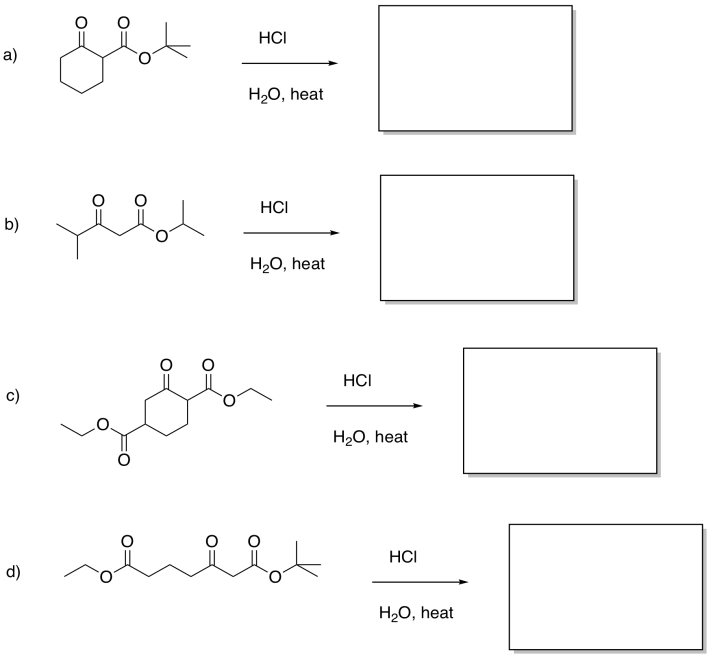
Problem CX7b.2.
Fill in the products of the following reactions.
Answers to selected problems are found here.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carboxyl Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry