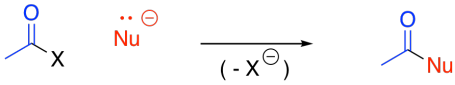
Reactivity in Chemistry
Substitution at Carboxyloids
CX7. Enolates: Claisen Condensation
Alkylmagnesium reagents, alkylcuprates and complex hydrides can all react with carboxyloids. When they do, a carbon or hydrogen nucleophile bonds to the carbonyl carbon, usually replacing the leaving group at that position.
Figure CX7.1. The general pattern of nucleophilic substitution at a carboxyloid.
Many of these reagents then add a second time, because replacing the leaving group with a hydrogen or a carbon group forms an aldehyde or a ketone, which can also react under the same conditions.
Another common carbon nucleophile is an enolate ion. Enolate ions can also react with carboxyloids, although not typically with amides.
Probably the most common enolate reaction involving carboxyloids is the reaction of esters. If a strong base is added to solution of ester, some of the esters will become deprotonated, forming enolate anions. These ions will be nucleophiles.
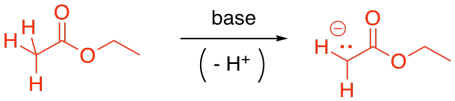
Figure CX7.2. The formation of an enolate nucleophile.
Some esters will remain protonated. These esters will be electrophiles. Donation of the enolate to the ester, with subsequent loss of the leaving group, leads to a beta-ketoester.
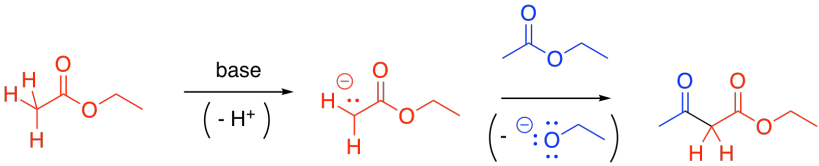
Figure CX7.3. A Claisen condensation.
You are familiar with the term "alpha-position". That's the position next to a carbonyl. The "beta-position" is the next one after the alpha position. In a beta-ketoester, there is a ketone in the beta position of the ester. The formation of a beta-ketoester from two esters is called a "Claisen condensation". The structural pattern formed contains R-C(O)-CH2-CO2R'.
In principle, this reaction could conceivably go backwards. The enolate ion could potentially be displaced by an alkoxide to get back to an ester and an enolate ion. That's because the enolate ion is a relatively stable ion, and a moderately good leaving group. However, that generally doesn't happen.
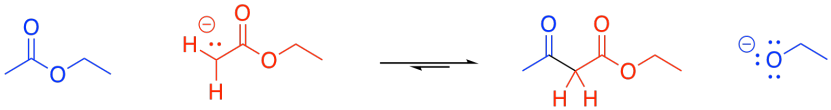
Figure CX7.4. The same compound appears as both nucleophile and electrophile.
Under basic conditions, the beta-ketoester is usually deprotonated, forming a particularly stable ion. This ion formation acts as a "thermodynamic sink" for the reaction, pulling it forward until all of the ester has been consumed. The reaction might still happen without this step, but yields are pretty low (below 50%).
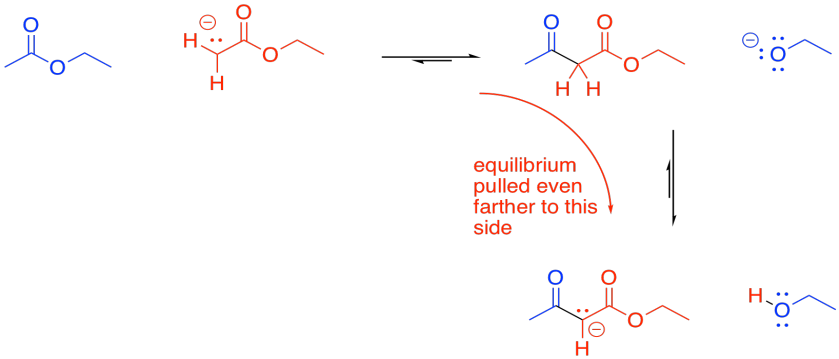
Figure CX7.5. High yields are obtained if there is a second α-proton to remove.
Problem CX7.1.
Show why the ion that results from deprotonation of the beta-ketoester is particularly stable.
Problem CX7.2.
FIll in the products of the following reactions.
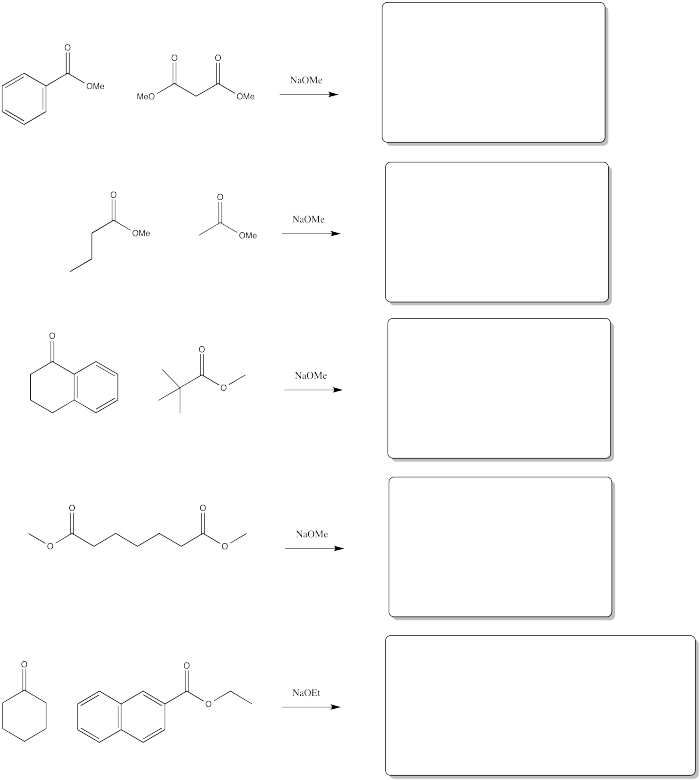
Problem CX7.3.
Predict the reactants needed to make these products via a Claisen, Aldol or Crossed Aldol reaction.
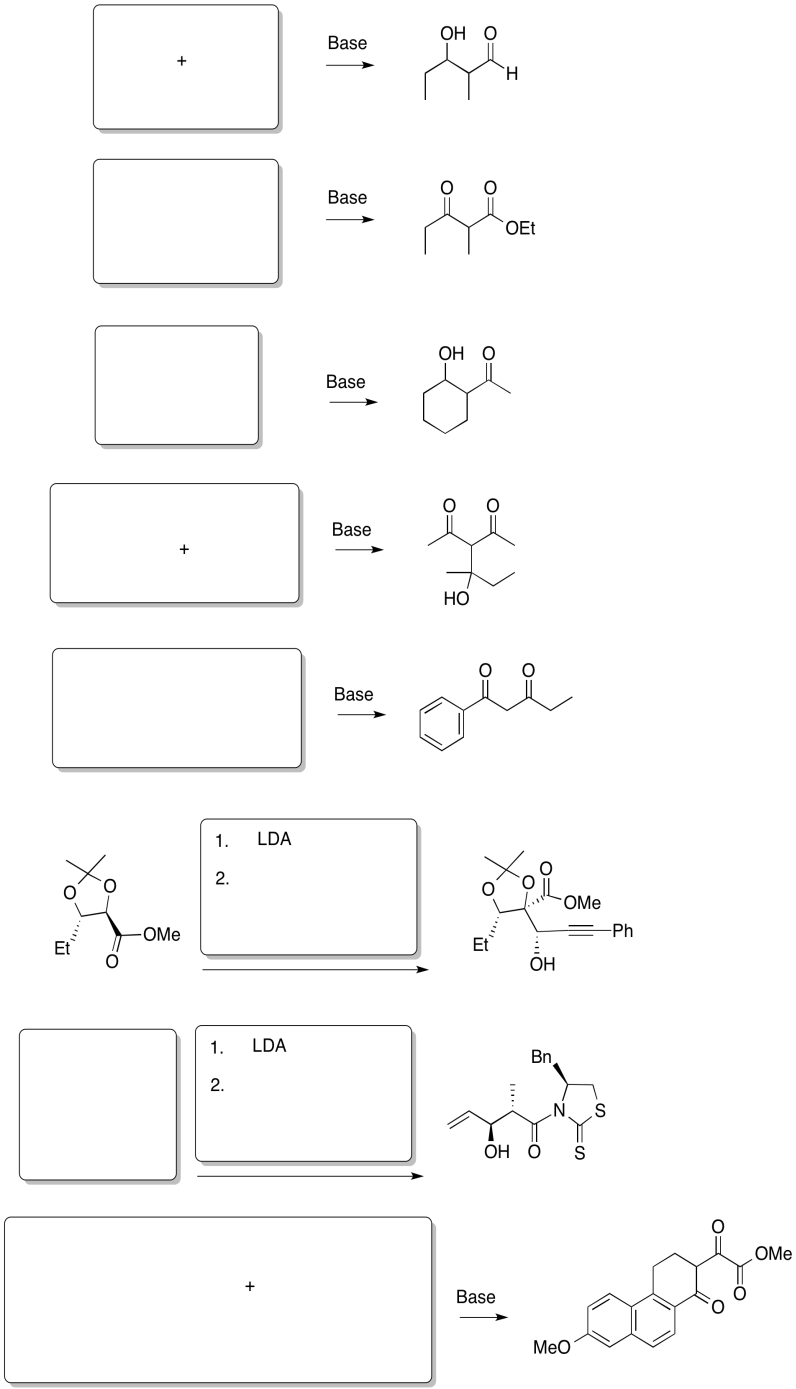
Answers to selected problems are found here.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carboxyl Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry