Part EA6. Insertion at Coordinated Alkenes (and the Reverse Reaction: Elimination)
Metal alkoxides can undergo 1,2-elimination (or beta-elimination) to give organic carbonyl compounds. This reaction is the reverse of a nucleophilic addition of a metal hydride to an organic carbonyl.
Figure EA6.1. Beta-elimination from a metal alkoxide.
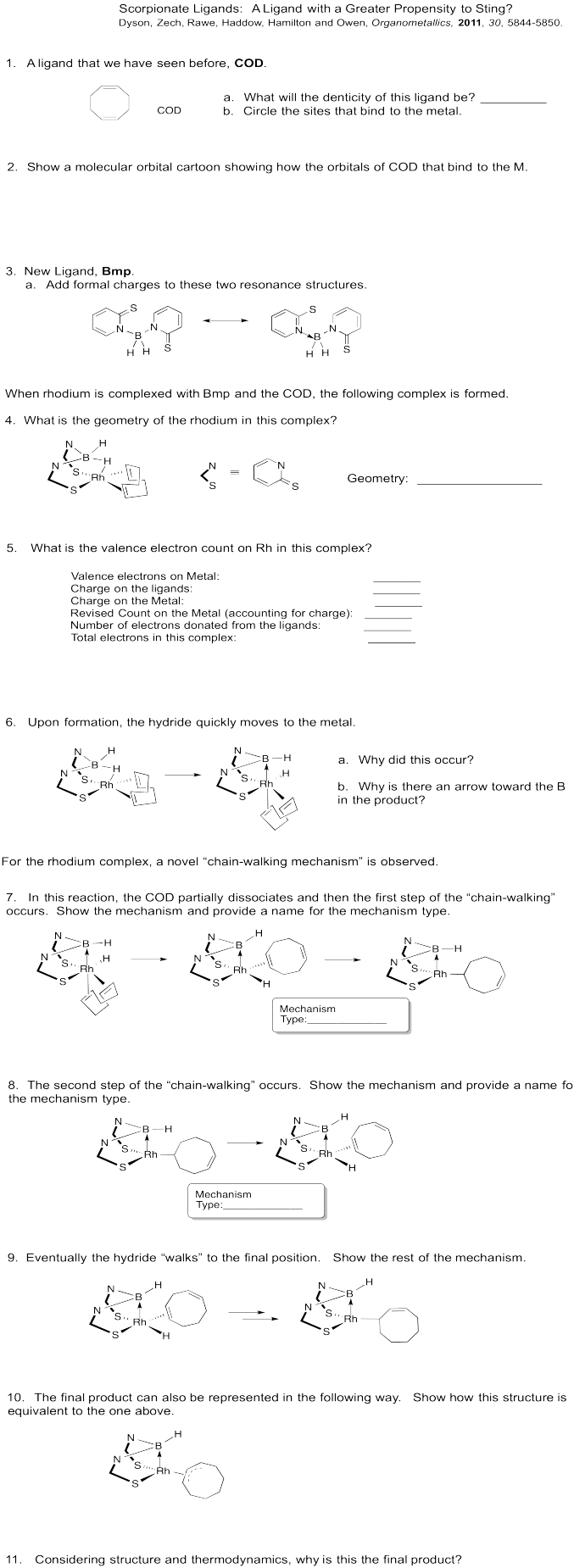
Metal alkyls can also undergo 1,2-elimination. In this case, an alkene is formed. Like the carbonyl compounds formed from 1,2-elimination, the alkene usually remains bound to the metal. It can dissociate to form the free alkene, though. For more information on alkene binding, take a look at this page.
Figure EA6.2. Beta-elimination from a metal alkyl complex.
These reactions are sometimes called "beta hydride eliminations", emphasizing that the hydrogen attached to the metal usually acts as a nucleophile. Thus, when the hydrogen transfers to the metal, it is forming a hydride.
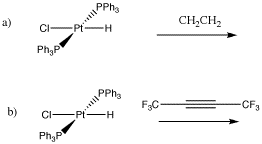
- Alkyl groups that are coordinated to metals can also undergo elimination.
- In order to undergo elimination, an alkyl group must have a hydrogen.
- When a metal alkyl undergoes elimination, it forms an alkene.
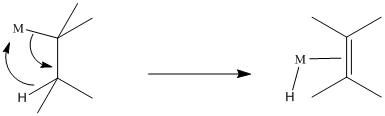
Problem EA6.1. Draw the elimination products in the following cases.
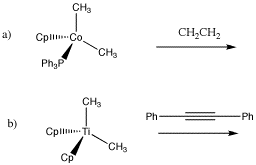
The reverse of a 1,2-elimination is a 1,2-insertion. Just like aldehydes and ketones, alkenes can undergo 1,2-insertions (also called beta hydride insertions). In terms of electrophiles and nucleophiles, this reaction is a little harder to imagine. However, we can still think of the hydride as a nucleophile. Maybe the alkene is an electrophile. Given that it is donating its pi electrons to the metal, we can think of it as "activated", a little bit like an activated carbonyl.
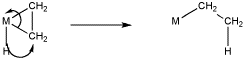
The formalisms of drawing a beta alkene insertion are tricky. If we use the metallacycle drawing of a bound alkene, it might look like this:
Figure EA6.3. 1,2-insertion of hydride into a coordinated alkene (shown using a metallacyclopropane formalism).
More often, bound alkenes are drawn as shown as in the picture below. In that case, we could try to show the pi bond forming a new carbon-metal bond. The bond between the metal and alkene on the picture to the left does not really stand for a separate pair of electrons in this case; it just stands for the pi bond donating to the metal.
Figure EA6.4. 1,2-insertion of hydride into a coordinated alkene (shown using a dative formalism).
- The reverse of elimination is insertion.
- A coordinated alkene is sometimes considered electrophilic because it is giving electrons to the metal.
- A coordinated alkene is activated, like a coordinated carbonyl compound.
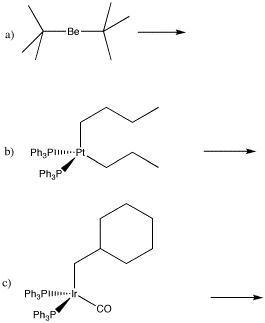
Problem EA6.2.
Draw the insertion products in the following cases.
Problem EA6.3.
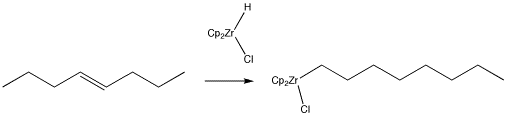
Alkenes can be converted into other compounds through the use of organometallic reagents, such as "Schwartz's reagent" (below). In this case, the resulting alkyl compound can easily be converted into a long-chain alkyl halide or alcohol through the addition of appropriate reagents. Provide a mechanism for the reaction shown below.
Problem EA6.4.
1,2-alkyl insertions and -eliminations are also known in a few cases, although they are much slower than 1,2-hydride insertions and -eliminations. Show the 1,2-insertion products for the following cases.
Problem EA6.5.
Problem EA6.6.
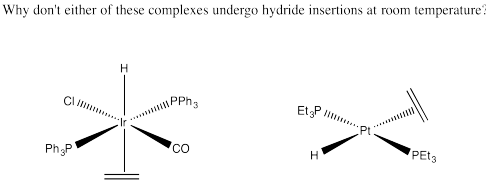
Problem EA6.7.
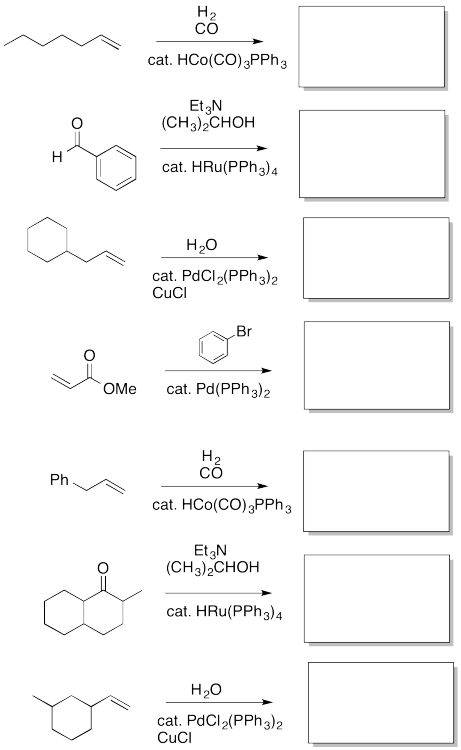
Fill in the missing insertion / elimination products.
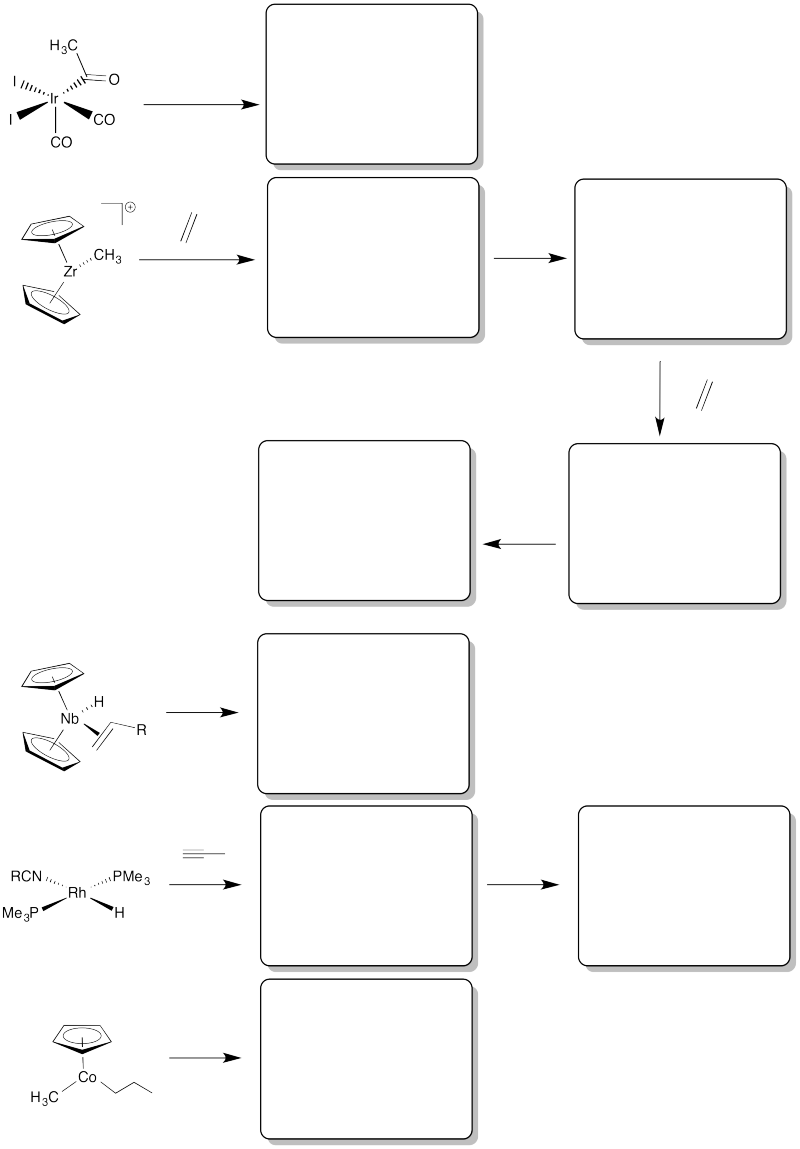
Problem EA6.8.
The following multi-part problem is based on an article in the primary research literature.