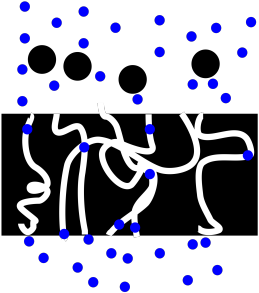
PM2. Filtration
There are a couple of very basic purification methods that are used routinely in the laboratory, and that are actually key steps in other methods. Filtration is one of them.
Suppose you have a mixture that contains both a solid and a liquid. There are a couple of ways you could separate one from the other. Decanting is one way. In decanting, the liquid is sitting nicely on top of the solid, so you can just pour off the liquid and leave the solid behind. However, usually that leaves some of the liquid behind in the solid, so you haven't completely separated them.
Filtration is a more thorough way of separating a solid from a liquid. The most familiar example might be a coffee maker. A coffee maker filters coffee from the ground coffee beans. The coffee falls through a filter paper, powered by gravity, and the coffee grounds remain on top of the filter paper.
Filtration relies on a porous material. In a porous material, there are pores or openings in the material that will allow small molecules pass through. You can think of the pores as tunnels. Small molecules, such as water and the organic molecules responsible for the properties of coffee, can easily move through the pores in the filter paper. Other materials, such as coffee grounds, cannot.

Figure PM2.1. A cartoon representing filtration.
In the laboratory, filtration can be done just as in the coffee filter. A filter paper is placed in a funnel and the mixture is poured in, and the liquid drips out under gravity. The liquid that drips through the filter paper is called the "filtrate".
Usually the funnel is a simple glass cone with a hole in the bottom, most often with a glass tube or stem leading from the hope to help guide the liquid to the vessel below. Sometimes, the funnel may have a flat bottom with holes in the bottom, or even a seemingly solid, but porous material. This last type is called a fritted funnel. The frit in the fritted funnel is porous material, just like filter paper, but it may be made of glass or plastic.
One of the advantages of a fritted funnel is that it is easy to use a vacuum to speed up filtration. By reducing pressure on the bottom side of the frit, the atmospheric pressure above the frit helps to push the solution through the frit more quickly.
The trouble is, frits can easily get clogged. Suppose there are particles suspended in the solution that are just small enough to get into the pores, but not small enough to move all the way through. They get stuck. Now we have a problem.
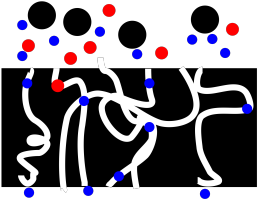
Figure PM2.2. A cartoon showing how a frit can get clogged during filtration.
The most common way to prevent this problem is to just put a piece of filter paper over the frit. The paper may prevent those particles from getting to the frit in the first place. The filter paper may get clogged in the same way, but you can just throw it out. Disposable filter paper may seem like a waste, but it is a much smaller waste than a disposable fritted funnel.
For really sticky situations, other filter aids include celite, a powder made from diatomaceous earth. A layer of celite is simple placed on top of the filter paper. Like the filter paper, the celite is disposable, but it protects the frit and allows the filtration to proceed more smoothly.
Note that filtration might allow isolation of either the solid phase or the liquid phase. In the former case, we would simply collect the solid that was held on the filter paper (we wouldn't use a filter agent like celite, which would contaminate the solid. The solid is usually allowed to dry before it is weighed and characterized.
If we are interested in the liquid, we might have more work to do. Maybe the liquid is pure, in which case we are finished. If it is a solution, we may wish to evaporate the solvent so that we could isolate the solute that we want.
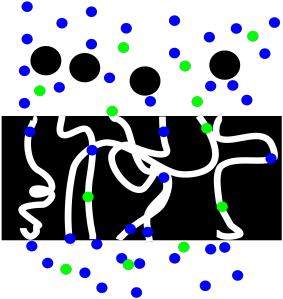
Figure PM2.3. A cartoon showing filtration of a solution.
Problem PM2.1.
In the following cases, indicate which compounds would be found on the filter paper and in the filtrate.
a) A mixture of sodium carbonate and heptanal is stirred in diethyl ether and filtered.
b) A mixture of lithium chloride and benzophenone is stirred in water and filtered.
c) A mixture of anthracene and potassium benzoate is stirred in water and filtered.
d) A mixture of ethylenediamine and tris(ethylenediamino)cobalt(III) chloride is stirred in diethyl ether and filtered.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to PurificationBack to Structure & Reactivity