Thermodynamics of the GTP-GDP-operated Conformational Switch of Selenocysteine-specific Translation Factor SelB. Alena Paleskava, Andrey L. Konevega and Marina V. Rodnina . The Journal of Biological Chemistry, 287, 27906-27912 (2012). doi: 10.1074/jbc.M112.366120 .
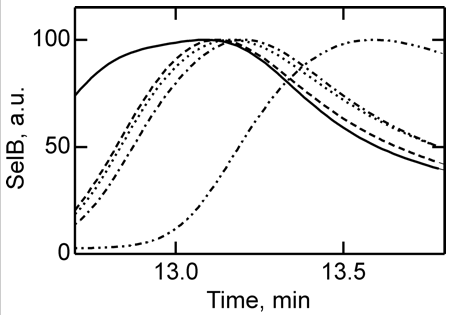
4. To further study the differences in SelB conformations upon binding of GTP, GDP, and nonhydrolyzable analogs, SelB and its complexes were separated by size-exclusion chromatography which separates on hydrodynamic volume (with larger molecules of the same shape eluting first). Figure 4 below shows the separation of apo form and nucleotide-bound forms of SelB by size-exclusion chromatography. Elution profiles of SelB·GTP (——), SelB·GTPγS (– – –), SelB·GDP (····), SelB·GDPNP (·–·–·–), and SelB (··–··–··–) were monitored by intrinsic tryptophan fluorescence (λexcitation = 280 nm, λemission = 355 nm). a. u., arbitrary units. Are these results consistent with the ITC results? Is the order of elution expect based on the results?

Answer: The apparent hydrodynamic radii of SelB in apo form and nucleotide-bound forms were significantly different, providing a clear separation of all five forms of SelB (Fig. 4). The order of elution follows the magnitude of conformational changes in SelB that were derived from the ITC data, with the complexes eluting in the order SelB·GTP, SelB·GTPγS, SelB·GDP, SelB·GDPNP, and SelB-apo. The differences between the GTP, GDP, and apo forms are particularly clear.
Note however, that the order of elution is not what was expected. If SelB:GTP had the largest negative ΔCp, and this is associated with the largest changes in surface area of the protein, you would expect that the SelB:GTP complex might have the smallest hydrodynamic radius and elute last from the column, not first. In lieu of crystal structures, which might shown a nonspherical shape that might justify the order of elution noted, we have to be satisfied that the separation did correlate with the experimental ΔCp values.