|
Cisplatin (structure shown below) is a potent
anticancer agent.
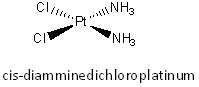
On entering a cell, a chloride (low concentration
inside a cell compared to outside) in cisplatin is replaced with water.
The water and remaining chloride are then replaced by ring Ns on adjacent
guanosine bases on the same strand. This bends the DNA. Two things can then
occur. Repair enzymes can remove the bound cisplatin and restore the
original structure. Alternatively, the bent structure leads to a
series of chemical events that lead to cell death in a process called
apoptosis.
|
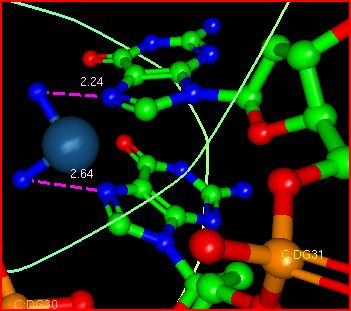
Cisplatin (Pt (gray),
NH3
(blue without Hs), spacefill) interacting
with N7s on adjacent guanosine bases (thick lines) on the same strand of double stranded
DNA. Note that the two N7s are NOT involved in H bonds with C
bases on the other DNA strand and hence are available in the narrowed
major grove to interact with cis-platin
Double stranded
DNA with cisplatin bound in the major grove: DNA backbone (green);
bases (light blue); bound cisplatin (ball and stick):
Pt (gray),
NH3
(blue with Hs).
The image below shows the interaction of cis-platin with the N7 atoms on adjacent G residues in one strand of the DNA. The resulting interaction causes a bend in the
dsDNA, compressing the major grove and expanding the minor
grove. The dsDNA at the binding site shows a transition from B to A dsDNA
structure and a bend of around 26 degrees.. The bend is reminiscent of the
effects on dsDNA on binding of the
TATA binding protein. The bending
probably facilitates the binding of proteins to the region which ultimately
leads to the toxic effects of the drug.