Chapter 4: Chemical Reactions
Moles, Molar Mass, and Avogadro's Number
To understand how substances react chemically, we need to be able to determine how many molecules of one substance reacts with another substance. In the lab we do not measure molecules directly but rather the mass of the reacting chemicals and products. Therefore we have to be able to convert between mass in grams and molecules.
I started off the a simple demonstration of 4 different fruits (a raisin, grape, apple, and grapefruit) in sealed boxes.
![]()
If each box contained the same number of pieces of fruit, then the box with the fruit with the heaviest piece of fruit would obviously weigh more. Converse, if the weights of the boxes were identical, then the box with the lightest piece of fruit would have the most pieces of fruit. If you knew the weight of each piece of fruit, you could determine the number of pieces of fruit in the box.
Now consider atoms like He, C, or Ti, and a molecule like sulfuric acid, H2SO4. I have arranged these in term of increasing atomic weight (or molecular weight for sulfuric acid). Hence if different boxes contained equal number of these different atoms or molecules, then the box with sulfuric acid would weight most, and the box with He would weigh least. Conversely, if the boxes weighed the same amount, the box containing the lightest atom, He, would contain more particles than the others.
The following statements are true and following in a logical sequence
It can be shown that the atomic weight of an atom of a pure element like Cu, Ti, etc, or the molecular weight of a molecule, both expressed in grams, contains 6.022 x 1023 atoms or molecules of that substance. Hence 12 g of 12C contains 6.022 x 1023 atoms while 98 g of sulfuric acid contain 6.022 x 1023molecules. 6.022 x 1023 is called Avogadro's number. The mass in grams of a substance that contains an Avogadro's number of particles is called the molar mass. It is the atomic (for atoms) or molecular weight (for molecules) expressed in grams. The "amount" of a substance that contains 6.022 x 1023 particles of that substance is 1 mole of that substance. You must be able to convert, using the factor-label method to convert between moles, molar mass, and number of molecules.
ANIMATION: Triangular Relationship of mass, molecules, and moles
It just so happens that 12.0 g of 12C has 6.022 X 1023 atoms. Too bad it is such a bizarre number. It would have been better if it were 1 billion, but there is nothing any of us can do about it. We define 1 mole of 12C as the mass of 12C that contains 6.022 X 1023 atoms., which , as described above, has a mass of 12.0 g. An atom of H has a mass 1/12 of carbon. (Look at the atomic weights) Therefore 6.022 X 1023 atoms of H would weight 1/12 of 12.0 g, or 1.0 g, which again happens to be the atomic weight of H expressed in grams. Likewise, H2O (molecular weight of 18.0) has a molar mass of 18.0 g. 18.0 g of water is 1 mole of water and contains 6.022 X 1023 molecules of water. Hemoglobin (MW = 64,000) has a molar mass of 64,000 g. 64,000 g of hemoglobin contains 6.022 X 1023 molecules of hemoglobin. By the way 1 mole of elephants would be 6.022 X 1023 elephants.
Writing and Balancing Chemical Equations
We then introduced the writing and balancing of chemical equations. The balanced equation below illustrates the important features of the equations
Au2S3 + 3 H2 ------> 2 Au + 3 H2S
This chemical equation can be translated into English as follows:
1 molecule of gold sulfide reacts with 3 molecules of hydrogen to produce 2 atoms of gold plus 3 molecules of hydrogen sulfide.
Both sides of a chemical equation, like a mathematical equation, can be multiplied by the same number. The table below shows various amounts of reactants and products that are obtained by multiplying the chemical equation by some number.
multiply by |
Au2S3
|
3 H2
|
2 Au
|
3 H2S
|
- |
1 molecule | 3 molecules | 2 atoms | 3 molecules |
2 |
2 molecules | 6 molecules | 4 atoms | 6 molecules |
12 |
12 molecules | 36 molecules | 24 atoms | 36 molecules |
OR |
1 DOZEN | 3 DOZEN | 2 DOZEN | 3 DOZEN |
6.02 X 1023 |
1(6.02 X 1023) molecules |
3 (6.02 x 1023) molecules |
2(6.02 x 1023) atoms |
3(6.02 x 1023) molecules |
OR |
1 mole molecules. | 3 moles molecule | 2 moles atoms | 3 moles molecule |
| which equals | 490 g | 6.0 g | 394 g | 102 g |
Please note the following:
Stoichiometry
The coefficients in front of balanced chemical equations tell us the number of moles of reactants that react to form a specific number of moles of products. The reaction stoichiometry is the quantitative relations between the amount of reactants consumed and products formed in chemical reactions as depicted in the balanced chemical equations. Stoichimetric relationship can be used to determine tells use that in the chemical reaction below :
Au2S3 + 3 H2 --> 2 Au + 3 H2S
that 490 g of Au2S3 reacts with 6 g H2 to give 394 g of Au and 192 g of H2S. (See table above). Simple proportions could be used to determine amounts of gold and hydrogen sulfide produced if the reactants were only at half the above concentration.
What would happen, however, if we had 490 g of gold sulfide and 100 g of hydrogen? Clearly not all the hydrogen could react. Hydrogen is in excess and some would be left after the reaction. Gold sulfide is the limiting reagent and the amount of products produced would be determined only by the amount of the gold sulfide, and not the hydrogen.
A simple demonstration of the concept of excess/limiting reagents is illustrated in the model below. You have two different 2D structures, a triangle and a square with a triangular indentation the same size of the triangle. You have 1 square and 4 triangles. The triangular indentation is lined with Velcro, and the triangle with the opposite type of Velcro. Now mix them together. Since only one triangle can fit into the indentation in the square, after mixing one square-triangle "complex" results and 3 triangles are left over. For an obvious chemical example, burn a 10 g piece of coal that is pure carbon in the air. Obviously oxygen in the air is in excess and the coal is limiting.
MODEL: LIMITING AND EXCESS REAGENTS
In another example, consider the reaction shown above in the table. If you had 490 g of Au2S3 and reacted it with 100 g of H2, not all the hydrogen would react. The above example shows that it can react with only 6 grams of hydrogen, leaving 94 grams unused. This is a consequence of the Law of Mass Conservation which is demonstrated in the table above. Remember the chemical equation gives the the ratio of the number of moles of the reactants the react and the ratio of the number of moles of product formed IN AN IDEAL REACTION WHEN ALL REACTANTS AND COMPLETELY CONVERTED TO PRODUCT WITH NO REMAINING REAGENTS LEFT OVER. In most laboratory situations or real-life problems, you are given a certain amount of reactants and want to know what the mass of the products would be if the limiting reagent (the reactant not in great excess) is completely converted to product.
Use the following general methods of solving stoichiometry problems. The critical components when doing such problems are:
Repeat until all units cancel except the desired unit to the right of the equal sign. Now time to take a WCB quiz.
![]()
WebCT Quiz: Select Stoichiometery 1 as the Quiz
REACTION TYPES
Think of the incredible number of chemical reactions going on around you and in you all the type. Each of your cells as you reading this is orchestrating thousands of different chemical reactions simultaneously. Since the advent of alchemy people have been making new types of compounds that never existed before. Our goal is now not to just balance chemical equations and determine how much reactant is consumed or product formed, but rather to predict what products might form given the reactants. Luckily, the myriad of different chemical reactions can be reduced to a few types. All of these were observed in Lab Experiment 3 in which copper was converted through a series of chemical reactions to other substances and ultimately back to copper. Several schemes have been developed to categorize chemical reactions. These include:
Charge transfer: Reactions involving making and breaking bonds and transfer charge between substances. If a proton is transferred from one substance to another, the reaction is called an acid/base reaction. An electron transfer is an oxidation/reduction reactions. Finally soluble ions of salts can be "transferred" towards each other in solution and form an insoluble solid. This reaction is called a precipitation reaction.
Composition Change: When reactants reagent to form products, they can rearrange in some familiar ways. A substance can displace another substance form a column. Two substances can combine to form one new substance or can decompose to from two or more substances. Substances can also exchange parts.
Driving Force: Some common driving forces for reactions are neutralization reactions (often involving formation of solvent water, gas evolution, solid formation, or formation of a less reactive product (such as an acid or base)
Summary of Chemical Reactions Classifications Schemes
Precipitation Reactions
Precipitation reactions occur when different aqueous solutions (usually of salts) are mixed, and a solid substance results. Remember solutions are homogeneous mixtures in which each part of the solution has the same composition. A solution can be colored (such as a blue copper sulfate solution) but it is always clear. Clear means that the solution is transparent, not cloudy, turbid, translucent, or opaque.
Most of the precipitation reactions that we will deal with involve aqueous salt solutions. Remember salts are compounds which consist of metal cations like Na+, Ca2+, Cu2+ (or the one nonmetal molecular ion that we have discussed, ammonium - NH4+) ionically bonded to nonmetal anions such as Cl-, (including molecular anions such as hydroxide - OH-, sulfate - SO42-, phosphate - PO43-, nitrate - NO3-, and carbonate - CO32-), dissolved in water. Salts can be divided into two types: those soluble in water, and those insoluble in water. You should know some simple solubility rules which will allow you to know which salts are soluble in water.
When salts dissolve in water, they seem to "melt away". What happened to the ions. An import clue comes from the fact that the resulting solution conducts electricity while pure water doesn't. We demonstrated this but putting a light bulb connected to two electrodes into water or a sodium chloride solution and connected it to an electrical outlet, as shown below.
ANIMATION: Do water and a solution of sodium chloride conduct electricity?
Before we can understand this, we need to have a quick review of electricity. As a water current is a flow of water, a current of electricity can be viewed as a flow of electrons. Consider our hypothetical copper wire. Copper is a metallic element, but does it exists as copper atoms in the wire? This can't be since atoms of any element except the Group VIII Nobel gas elements are reactive. Something must be holding the copper particles together and cause the wire to be a good conductor of electricity (i.e. it can carry a current of electrons). In fact, the copper particles don't exist as atom but rather as positive ions which have given up an outer shell electron. These copper ions surrounded by a sea of mobile electrons account for the stability of the solid copper and the fact that it can conduct electricity - which is a flow of charged particles - in this case of electrons.
Why does a solution of NaCl conduct a current? Somehow when NaCl dissolves, it must formed individually charged particles. We know the NaCl consists of an array of Na+ and Cl- ions. If when NaCl dissolves, it separates into individual molecules of neutral NaCl, then the solution would not contain particles with an overall net charge. This suggests that the individual ions which comprise a "molecule" of the NaCl separate from each other -i.e. they dissociate - in water. As we shall see in the next chapter, the O (slightly negative) on water interacts with the metal ion (positively charged cation), while the H (slightly positive) on water interacts with the nonmetal ion (negatively charged anion), separating the two ions. Charge particles are required for a flow of electric current. If the molecules of salt didn't separate into ions, the smallest particles in solution would be neutral salt molecules. NaCl and other soluble salts are strong electrolytes.
Precipitation reactions occur when different salt solutions are mixed, which result in the formation of an insoluble salt, or precipitate. When precipitation occurs, the cation of one of the soluble salts interacts with the anion of the other soluble salt to form an insoluble salt. The other ions which remain soluble are called spectator ions. molecular, ionic, and net ionic equations. Consider the following example when aqueous solutions of NaCl and AgNO3 are added to each other. A white cloudy precipitate forms. What is the likely product that formed a precipitate? Consider all the ions in solution. If the positive ions approached each other, they would repel, so we can't form compounds like NaAg. The same is true of the negative ions. Ions of opposite charge can attract each other so we could reform NaCl and AgNO3. However, these are soluble salts and would immediately dissociate again into individual ions. The other possibilities are AgCl and NaNO3. The later would likewise dissociate since we know that Na and NO3 are soluble. The only alternative is AgCl. We can write a series of chemical equations to explain these interactions:
molecular equation: write down the species as molecules as below-
NaCl (aq) + AgNO3 (aq) --> AgCl (s) + NaNO3 (aq) where the (aq) for aqueous implies that the salt is in solution as is soluble.
ionic equation: write down the species as they actually occur in the solution:
Na+(aq) + Cl-(aq) + Ag+(aq) + NO3-(aq) --> AgCl(s) + Na+(aq) + NO3-(aq)
net ionic equation: remove identical ions that appear on both sides fo the ionic equation that act as "spectators" in the reaction. They are called spectator ions.
Ag+(aq) + Cl-(aq) --> AgCl(s)
ANIMATION - Formation of AgCl precipitate
![]()
WCB Quiz:. Precipitation Reactions - underconstuction
(note: until the WCB quiz program is working correctly, I will just insert the quiz here and email use the answers. Obviously, these ungraded quizzes are for your own good, so it makes no sense to look up the answers first.)
WCB Quiz H: Precipation Reactions - Answers
Breaking Bonds: Charge transfer reactions - Acid/Base and Oxidation/Reduction (Redox) Reactions
Chemical reactions involve making and breaking bonds. So far we have considered making and breaking ionic bonds between ions in aqueous salt solutions. Now we want to discuss making and breaking covalent bonds. When we break a covalent bonds, there are three ways we can separate the two atoms involved in the bond. Consider for example a C-X bond. If when the bond is broken, the electrons are left with C, C takes on a negative charge and is called a carbanion. If the electrons leave with X, then C is left with a positive charge and is a carbocation. If one electron stays with each atoms, the C has no charge, but does not have an octet. The carbon is called a free radical.
When the two electrons in a bond between X and H remain with X, a proton (H without its electron) is cleaved from the molecule. An isolated proton is very reactive and will bind to another molecule which can accept it. A reaction in which a proton is donated to another molecule is called an acid/base reaction. If electrons are transferred from one substance to another, the reaction is called a oxidation/reduction (redox reaction). We will first consider acid/base reactions.
Acid/Base Reactions
First, let's backtrack and consider what types of substances, when dissolved in water, allow for the conductance of an electric current. Remember, for the light to light, ions must be present in solution. Previously, we saw that an aqueous NaCl solution conducted electricity. Obviously the ionic bonds holding Na and Cl must be broken when solid NaCl dissolves in water which results in separate Na+ and Cl- ions. What about MgCl2? Of course it must since it is a salt, held together with ionic bonds, that dissolves in water and forms ions. What about AgNO3? Likewise this should as well since it is also a salt of the metal Ag and the polyatomic ion NO3- which separate into individual Ag+ and NO3-ions. (Remember the N and O atoms in this polyatomic or molecular ion, which are held together by covalent bonds, stay together). Now let's consider compounds not held together by ionic bonds. Consider for example, methanol, [CH3OH(l)] and glucose [C6H12O6(s)]. There structures are shown below:
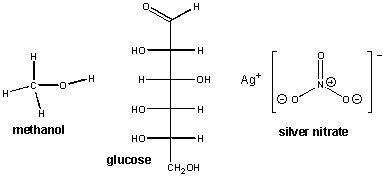
When these substances were dissolved in water, no light was observed. This implies that were no ions in solution. From these examples, we can tentatively conclude that covalently bonded species don't dissolve in water to form ions. This makes sense in that pure H2O itself, a molecule held together by polar covalent bonds, did not conduct electricity in our experiment.
Now consider one last example: HCl(aq), made by dissolving HCl(g) in water. In this demonstration, a bright light was observed. The only way we could get a light in this solution is if charged ions were present. Yet HCl is a neutral molecule. How could such charged ions be produced. The best possibility is that the H-Cl bond was broken and the electrons went to Cl making it Cl- with 8 outer shell electrons. We saw a similar situation when salts dissolved and the ions that comprised the salt dissociated (or separated from each other) to form individual ions. The case of HCl is different. It is a covalently bonded molecule - i.e. it is not made up of ions. However, when the HCl bond breaks, ions are formed. We call this process ionization (compared to dissociation of ionically bonded salts). The H would be left with no electrons and becomes H+ which is simple an isolated proton. Classes of molecules like HCl that are bonded covalently yet seem to give up a proton in water when dissolved are called acids. Earlier definitions of acids were that they formed H+ in solutions.
We now know that the the bond between H and Cl is not just severed by itself. Another species in solution - in this case water - attacks the HCl and pulls off the proton. The HCl donates a proton, our definition of an acid, and the water accepts a proton. A proton acceptor is called a base. This concept of acids and bases was developed by Bronsted.
The acid/base reaction discussed above can be summarized by the equation:
HCl(aq) + H2O(l) ---> H3O+(aq) + Cl-(aq).
Again, H+ ions aren't just formed in solution. Rather an agent, water in this case, pulls the H ion off to form H3O+ . Just as in redox reactions, electrons are not simply lost when a substance gets oxidized, but rather are "pulled off" by a oxidizing agent which gets reduced in the process. The agent that pulls off the proton (H+) from HCl is water. The agent that donates protons (HCl) is an acid, the agent that accepts protons (H2O) is a base. Bases must have lone pair electrons so they can accept a proton.
ANIMATION: Reaction of HCl and H2O.
In the reaction: HCl(aq) + H2O(l) ---> H3O+(aq) + Cl-(aq)
If you look at the products, you could imagine they could also react in a reverse of the original reaction to produce the original reactants.
H3O+ + Cl- ---> HCl(aq) + H2O(l)
In this reaction:
Why doesn't this reverse reaction also occur? (Didn't we discuss this with redox reactions above?) It actually does to an extremely small extent we can barely measure any undissociated HCl. You could actually envision the original reaction as reversible:
HCl(aq)A + H2O(l)B <===> H3O+(aq)A + Cl-(aq)B
where A indicates which reactants/products are potential acids in the forward and reverse reactions, and B indicates potential bases. Which way does this reaction go? We will discuss this in more detail in the next chapter and next semester, but suffice it to say, the reaction goes in the direction from the stronger acid and base to the weaker. In the above example, HCl(aq) is the stronger A and H2O(l) the stronger B. You can't predict from looking at the possibilities, but in the next chapter and next semester we will discuss how you can determine the relative strengths of acids and bases by knowing their Lewis structures and from data in tables. Strong acids such as nitric acid, sulfuric acid, hydrochloric acid are essentially completely ionized - they are strong electrolytes.
The simplest kind of acid base reaction that we experience often in the lab is that of a strong acid with a strong base. For us, the strong bases are those that contain OH- ions. Hence
HCl(aq)A + NaOH(aq)B <===> H2O (l)A + NaCl(aq)B - molecular eq.
H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq)
<===> H2O (l) + Na+(aq) + Cl-(aq)
ionic equation
H+(aq)+ OH-(aq) <===> H2O (l) net ionic equation
Note: a strong acid reacts with a strong base to form a salt and water - the acid and base are neutralized.
![]()
WCB Quiz: J. Acid/Base Reactions
(note: until the WCB quiz program is working correctly, I will just insert the quiz here and email use the answers. Obviously, these ungraded quizzes are for your own good, so it makes no sense to look up the answers first.)
WCB Quiz: J. Acid/Base Reactions Answers
Summary: Whats in the Beaker - Solubility, Dissociation, and Ionization
Oxidation/Reduction (Redox) Reactions
We have just discussed acid/base reactions, which involve proton transfer. Now we will discuss anothe kind of charge transfer, electron transfer or oxidation/reduction reactions. In oxidation/reduction reactions, there is a transfer of charge - an electron - from one species to another. Oxidation is the loss of electrons and reduction is a gain in electrons. (Remember Leo Ger - Lose of electrons oxidation; Gain of electrons reduction or Oil Rig - Oxidation involves loss, Reduction involves gain - of electrons.) These reactions always occur in pair. That is, an oxidation is always coupled to a reduction. When something gets oxidized, another agent gains those electrons, actiing as the oxidizing agent, and gets reduced in the process. When a substance gets reduced, it gains electrons from something that gave them up, the reducing agent, which in the process gets oxidized.
Reactions in which a pure metal reacts with a substance to form a salts are clearly oxidation reactions. Consider for example the reaction of sodium and chlorine gas.
2Na(s) + Cl2 -----> 2NaCl(s)
Na is a pure metal. Although we discussed that it really exists as copper ions and ions surrounded by a sea of electrons, consider it for our purposes elemental Na, which has a formal charge of 0. Likewise, Cl2 is a pure element. To determine the charge on each Cl atoms, we divide the two bonded electrons equally between the two Cl atoms, hence assigning 7 electrons to each Cl. Hence the formal charge on each Cl is 0. In a similar fashion we can determine the oxidation number of an atom bonded to another atom. We can assign electrons to a bonded atoms, compare that number to the number in the outer shell of the unbonded atoms, and see if there is an excess or lack. The other substance must be getting reduced. In these cases, the same number of electrons get assigned to each atoms as when we are calculating formal charge. Hence the oxidation numbers are equal to the formal charge in these examples. Clearly, Na went from an oxidation number and formal charge of 0 to 1+ and Cl from 0 to 1-. Therefore, Na was oxidized by the oxidizing agent Cl2, and Cl2 was reduced by the oxidizing agent Na.
Lets consider other similar redox reactions:
2Mg(s) + O2(g) ----> 2MgO(s)
Fe(s) + O2(g) ---> Fe oxides (s)
C(s) + O2(g) ----> CO2(g)
In the first two reactions, a pure metal (with formal chargess and oxidation numbers of 0) lose electrons to form metal oxides, with positive metal ions. The oxygen goes form a formal charge and oxidation number of 0 to 2- and hence is reduced.
What about the last case? Each atom in both reactants and products has a formal charge of 0. This reaction, a combustion reactions with molecular oxygen, is also a redox reaction. Where are the electrons that are lost or gained? This can be determined by assigning the electrons in the different molecules in a way slightly different than we did with formal charge. For shared (bonded) electrons, we give both electrons in the bond to the atom in the shared pair that has a higher electronegativity. Next we calculate the apparent charge on the atom by comparing the number of assigned electrons to the usual number of outer shell electrons in an atom (i.e. the group number). This apparent charge is called the oxidation number. When we use this method for the reaction of C to CO2, the C in carbon dioxide has an oxidation number of 4+ while the two oxygens have an oxidation number of 2- . Clearly, the C has "lost electrons" and has become oxidized by interacting with the oxidizing agent O2. as it went from C to CO2. If the atoms connected by a bond are identical, we split the electrons and assign one to each atom. In water, the O has an oxidation number of 2- while each H atom has an oxidation number of 1+. Notice that the sum of the oxidation numbers of the atoms in a species is equal to the net charge on that species.
What we have done is devise another way to count the electrons around an atom and the resulting charges on the atoms. See the animation below to review electron counting, and the 3 "types of charges" - partial charges, formal charges, and now oxidation numbers.
ANIMATION: Counting electrons and determining "charge" on an atom.
Consider an O-X bond, where X is any element other than F or O. Since O is the second most electronegative atom, the two electrons in the O-X bond will be assigned to O. In fact all the electrons around O (8) will be assigned to O, giving it always an oxidation number of 2-. This will be true for every molecule we will study this year except O2 and H2O2 (hydrogen peroxide). Now consider a C-H bond. Since C is nearer to F, O, and N than is H, we could expect C (en 2.5) to be more electronegative than H (en 2.1). Therefore, both electrons in the C-H bond are assigned to C, and H has an oxidation number of 1+. This will always be true for the molecules we study, except H-H. A quick summary of oxidation numbers shows that for the molecules we will study:
Notice in each of the reactions above, oxygen is an oxidizing agent. Also notice that in each of these reactions, a pure element is chemically changed into a compound with other elements. All pure, uncharged elements have formal charges and oxidation numbers of 0. When they appear as compounds in the products, they must have a different oxidation number. The disappearance or appearance of a pure element in a chemical reaction makes that reaction a redox reaction.
Now lets consider a more complicated case - the reaction of methane and oxygen to produce carbon dioxide and water:
CH4 + O2 -----> H2O + CO2.
Since H has an oxidation # of 1+, the oxidation # of C in CH4 is 4-, while in CO2 it is 4+. Clearly C has been oxidized by the oxidizing agent O2. O2 has been reduced to form both products.
Now consider a series of step-wise reactions of CH4 ultimately leading to CO2
.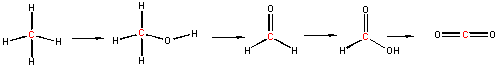
You should be able to determine that the oxidation numbers for the central C in each molecule are 4-, 2-, 0, 2+, and 4+ as you proceed from left to right, and hence represent step-wise oxidations of the carbon. Stepwise oxidations of carbon by oxidizing agents different than O2 are the hallmark of biological oxidation reactions. Each step-wise step releases smaller amounts of energy, which can be handled by the body more readily that if it occurred in "one step", as indicated in the combustion of methane by O2 above.
You may have learned in a previous course that in oxidation reactions, there is an increase in the number of X-O bonds, where X is some atom. Alternatively, it also involves the decrease of X-H bonds. Reduction would be the opposite case - decreasing the number of X-O bonds and/or increasing the number of X-H bonds. This rule applies well to the above step-wise example. Consider, however, the following reaction:
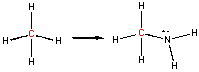
In this example, the C in methane has an oxidation # of 4- while in the product it is 2-. Once again, the C has been oxidized, however, the number of bonds to O has not increased. This shows the importance of being able to calculate an oxidation number to determine if a redox reaction has occurred. Where is the loss of electrons? It comes about since C is now bonded to a more electronegative atom (N), which withdraws electron density form the C.
Redox reactions are common in nature. Some common redox reactions are reactions that occur in batteries, when metals rust, when metals are plated from solutions, and of course combustion of organic molecules such as hydrocarbons (like methane and gasoline) and carbohydrates (like wood). A simple redox reaction that leads to the plating or deposition of a pure metal from a solution of that metal is shown below.
Cu(s) + 2Ag+(aq) ---> Cu2+(aq) + 2Ag(s)
In this reaction, pure silver metal - Ag(s) is plated on the surface of Cu(s) In this reaction:
If you look at the products, you could imagine they could also react in a reverse of the original reaction to produce the original reactants.
Cu2+(aq) + 2Ag(s) ---> Cu(s) + 2Ag+(aq)
In this reaction, pure copper metal - Cu(s) would be plated on the surface of the Ag(s). In this reaction:
Why doesn't this reverse reaction also occur? It actually does to a small extent. You could actually envision the original reaction as reversible:
Cu(s)RA + 2Ag+(aq)OA <===> Cu2+(aq)OA + 2Ag(s)RA
where RA indicates which reactants/products are potential reducing agents in the forward and reverse reactions, and OA indicates potential oxidizing agents. Which way does this reaction go? We will discuss this in more detail next semester, but suffice it to say, the reaction goes in the direction from the strongest oxidizing and reducing agents to the weakest. In the above example, Cu(s) is the stronger RA and Ag+ is the stronger OA. You can't predict from looking at the possibilities, but next semester we will discuss how you can determine the relative strengths of OA's and RA's from tables.
Consider this reaction from Lab 3 when you added solid Zn to the blue copper sulfate solution to produce pure Cu(s):
Zn(s) + Cu2+(aq) ---> Zn2+(aq) + Cu(s)
This reaction can be thought of as 2 half-reactions which can be added together to get the top reaction:
Zn(s) + ---> Zn2+(aq) + 2e-
Cu2+(aq) + 2e- ---> Cu(s)
Image if you tried to separate the reactions into two beaker, one containing Zn(s) and the other Cu2+. Obviously the reactions could not occur. However, if we connected the two beakers with a wire (which would allow electrons to flow from Zn(s) to Cu2+(aq), then the reactions can occur. If we put a voltmeter or light bulb in between the two beakers, a voltage is recorded or the light bulb will light. We have made these redox reactions into a battery.
Redox Reactions - Voltage Cell and Battery
![]()
(note: until the WCB quiz program is working correctly, I will just insert the quiz here and email use the answers. Obviously, these ungraded quizzes are for your own good, so it makes no sense to look up the answers first.)
WCB Quiz I: Redox Reactions - Answers
Summary of Reactions
Typical characteristics of these reactions are shown below:
Use those simple rules to classify simple reactions. Reactions involving acids can be tricky since their presence as a reactant does not imply that the reaction is an acid/base reaction, UNLESS A BASE IS PRESENT. This is also true of bases like OH- which can be a base or a precipitating agent. Tricky examples that we discussed include:
Similarities in Reactions:
As there is a continuum in bond types, from ionic (no sharing of electrons to nonpolar covalent with a complete sharing of electrons), there is a continuum in
The truth is that even with insoluble salts, there are small concentrations of dissolved ions in solution - too small to allow our light to light. Likewise, H2O can react with itself in an acid/base reaction to produce low concentrations of ions (again not enough to light a light), as shown below
H2O + H2O <==> H3O+ + OH-
This reaction is an autoionization of water in which water acts both as an acid and base.
The Transformation of Copper
In lab 3 we transformed pure copper foil through a series of chemical intermediates back into pure copper again. Hopefully you were awed by the amazing chemical transformations that took place. Click on the underlined reactions below .to relive your laboratory experiences. Then take the WCB to see how well you understood the reactions.
![]()
WCB Quiz K: Copper Reactions - Answers
Putting it all together
Our goal is to be able to predict the products of a reactants given the products. We have studied 3 types of chemical reactions. Many, but not all, chemical reactions can be classified as one of these. In the link below you will find a series of reactions. Predict the type of reactions and the probable products. Finish the molecular equations and balance the equations. I'll put the answers on later.
TOP TEN LIST OF MISTAKES ON NAME THAT PRODUCT
Hints when trying to predict products: