Biochemistry Online: An Approach Based on Chemical Logic

Structure & Reactivity in Chemistry
Metabolic Pathways
MP1. An Overview of Metabolic Pathways - Catabolism
Henry Jakubowski
Biological cells have a daunting task. They must carry out 1000s of different chemical reactions required to carry out cell function. These reactions can include opposing goals such as energy production and energy storage, macromolecule degradation and synthesis, and breakdown and synthesis of small molecules. All of these reactions are catalyzed by proteins and RNAs enzymes whose activities must be regulated, again through chemical reactions, to avoid a futile and energy wasting scenario of having opposing pathways functioning simultaneously in a cell.
Metabolism can be divided into two main parts, catabolism, the degradation of molecules, usually to produce energy or small molecules useful for cell function, and anabolism, the synthesis of larger biomolecules from small precursors.
CATBOLISM: Catabolic reactions involve the breakdown of carbohydrates, lipids, proteins, and nucleic acids to produce smaller molecules and biological energy in the form of heat or small thermodynamically reactive molecules like ATP whose further degradation can drive endergonic process such as biosynthesis. Our whole world is reliant on the oxidation of organic hydrocarbons to water and carbon dioxide to produce energy (at the expense of releasing a potent greenhouse gas, CO2). In the biological world, reduced molecules like fatty acids and partially oxidized molecules such as glucose polymers (glycogen, starch), as well as simple sugars, can be partially or fully oxidized to ultimately produce CO2 as well. Energy released from oxidative reactions is used to produce molecules like ATP as well as heat. Oxidative pathways include glycolysis, the tricarboxylic acid cycle (aka Kreb's cycle) and mitochondrial oxidative phosphorylation/electron transport. To fully oxidize carbon in glucose and fatty acids to carbon dioxide requires splitting C-C bonds and the availability of series of oxidizing agents that can perform controlled, step-wise oxidation reactions, analogous to the sequential oxidation of methane, CH4 to methanol (CH3OH), formaldehyde (CH2O) and carbon dixoxide.
-
Glycolysis: This most primitive of metabolic pathways is found in perhaps all organisms. In glycolysis, glucose (C6H12O6), a 6C molecule, is split (or lysed) into two, 3C carbon molecules, glyceraldehyde-3-phosphate, which are then partially oxidized under anaerobic conditions (without O2) to form two molecules of pyruvate (CH3COCO2-). Instead of the very strong oxidizing agent, O2, a weaker one, NAD+ is used, which is reduced in the process to form NADH. Since none of the carbon atoms is oxidized to the state of CO2, little energy is released compared to the complete oxidation to CO2. This pathway comes to a screeching halt if all cellular NAD+ is converted to NADH as NAD+ is not replenished by the simple act of breathing as is the case with O2 in aerobic oxidation. To prevent the depletion of NAD+ from inhibiting the cycle and to allow the cycle to continue under anaerobic conditions, excess NADH is reconverted to NAD+ when the other product of glycolysis, pyruvate is converted to lactate by the enzyme lactate dehydrogenase. Glycolysis occurs in the cytoplasm of the cell.
Figure: Summary of Glycolysis
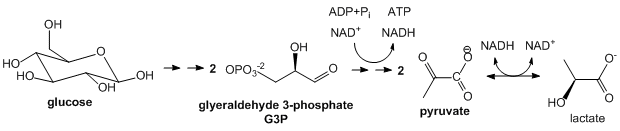
-
Tricarboxylic Acid (Kreb's) Cycle: The TCA cycle is an aerobic pathway which takes place in an intracellular organelle called the mitochondria. It takes pyruvate, the incompletely oxidized product from glycolysis, and finishes the job of oxidizing the 3C atoms all the way to CO2. First the pyruvate moves into the mitochondria where is is oxidized to the 2C molecule acetylCoA with the release of one CO2 by the enzyme pyruvate dehydrogenase. The acetyl-CoA then enters the TCA cycle where two more CO2 are released. As in glycolysis, C-C bonds are cleaved and C is oxidized by NAD+ and another related oxidizing agent, FAD. What is very different about this pathway is that instead of being a series of linear, sequential reactions with one reactant (glucose) and one product (two pryuvates), it is a cyclic pathway. This has significant consequences since if any of the reactants within the pathways becomes depleted, the whole cyclic pathway can slow down and stop. To see how this happens consider the molecule oxaloacetate (OAA) which condenses with acetyl-CoA to form citrate (see diagram below). In this reaction, one OAA is consumed. However, when the cycle returns, one malate is converted to OAA so there is no net loss of OAA, unless OAA is pulled out of the TCA cycle for other reactions, which happens.
Figure: Pyruvate Dehydrogenase (mitochondrial) and the TCA Cycle
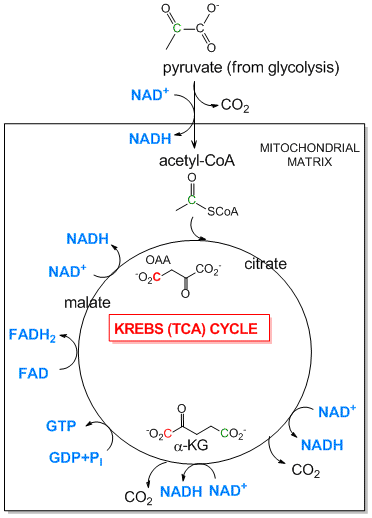
-
Mitochondrial Oxidative Phosphorylation/Electron Transport: The TCA cycle accomplishes what glycolysis didn't, that is the cleavage of all C-C bonds in glucose (in the form of pyruvate and acetyl-CoA, and the complete oxidation of all C atoms to CO2. Yet two problem remains. The pool of oxidizing molecules, NAD+ and FAD get converted to their reduced forms, NADH and FADH2. Unless NAD+ and FAD are regenerated, as was the case in anaerobic conditions when pyruvate gets converted to lacate, the pathway would again come to a grinding halt. In addition, not much ATP is made in the cycle (in the form of a related molecule GTP). Both these problems are resolved as the resulting NADH and FADH2 formed are reoxidized by mitochondrial membrane enzyme complexes which pass electrons from the oxidized NADH and FADH2 to increasingly potent oxidizing agents until they are accepted by the powerful oxidant O2,which is converted reduced to water. The net oxidation of NADH and FADH2 by dioxygen is greatly exergonic, and the energy released by the process drives the synthesis of ATP from ADP and Pi by an mitochondrial enzyme complex, the F0F1ATPase.
Figure: Mitochondrial Electron Transport/Oxidative Phosphorylation
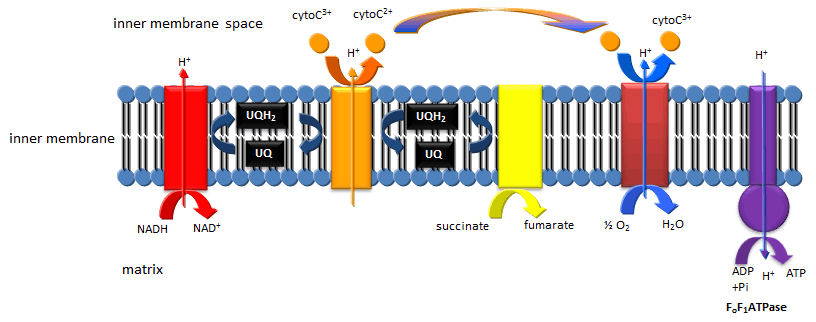
Feeder Pathways: Other catabolic pathways produce products that can enter glycolysis or the TCA cycle. Two examples are given below.
- Complex carbohydrates: In mammals, the major carbohydrate storage molecule is glycogen, a polymer of glucose linked a1-4 with a1-6 branches. The terminal acetal linkages in this highly branched polymer is cleaved sequentially at the ends not through hydrolysis but through phosphorolysis to produce lots of glucose-1-phosphate which can enter glycolysis.
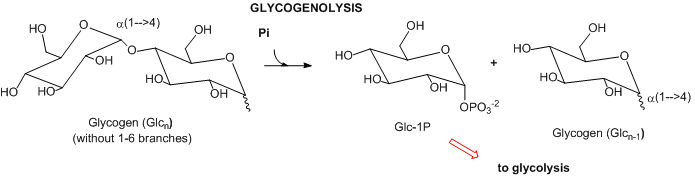
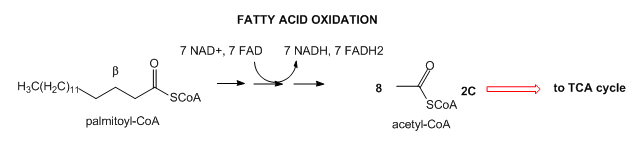
- Lipids: Lipids are stored mostly as triacylglycerides in fat cells (adipocytes). When needed for energy, fatty acids are hydrolyzed from the glycerol backbone of the triacylglyceride, and send into cells where they broken down in an oxidative process to form acetyl-CoA with the concomitant production of lots of NADH and FADH2. These can then enter the mitochondrial oxidative phosphorylation/electrons transport system, which produces, under aerobic conditions, lots of ATP.
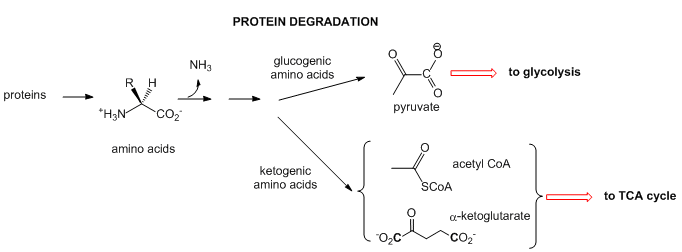
- Proteins: When intracellular proteins get degraded, they from individual amino acids. The amine N is lost as it enters the urea cycle. The rest of some amino acid structures can be ultimately converted to acetyl-CoA or keto acids (like alpha-ketoglutarate- a-KG) that are TCA intermediate. These amino acids are called ketogenic. Alternatively, some amino acids, after deamination, are coveted to pyruvate which can either enter the TCA cycle or in the liver be used to synthesize glucose in an anabolic process. These amino acids are called glucogenic. Chemical reactions such as these can be used to replenish intermediates in the TCA cycle which can become depleted as they are withdraw for other reactions.
Now on to anabolic reactions.
Navigation
| Reactivity Index | MP0. Metabolic Pathways - Main Menu |
| Structure & Reactivity Web Materials | Biochemistry Online: Table of Contents |

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.