Biochemistry Online: An Approach Based on Chemical Logic

Structure & Reactivity in Chemistry
Metabolic Pathways
MP5. Regulation of Metabolic Pathways B: Which Enzymes Are
Optimal for Regulation?
Henry Jakubowski
All proteins are ultimately regulated, if only by modulating the rates of their synthesis and degradation. However, some enzymes positioned at key points in metabolic pathways are ideal candidates for regulation, as their activity can affect the output of entire pathways. These enzymes typically have two common characteristics, they catalyze reactions far from equilibrium and they catalyze early committed steps in pathways.
A. Regulation of enzymes for reactions not at equilibrium:
The optimal enzymes for regulation are those at the beginning of pathways and that carry out thermodynamically favored reactions. Why is the latter so important? These enzymes control the flux of metabolites through pathway, so to understand their regulation we can use the analogy of flow (or flux) of water from one container to another. Let's say you wish to fill a swimming pool at any desired height you wish and you have two ways to do so (see figure below). You could open a valve that controls the flow from your towns water tower to the pool. In this the reaction (flow of water) is energetically (thermodynamically) favored given the difference in height of the water levels and the potential energy difference between the two. Even though flow (or flux) is cleared flavored, you can regulate it, from no flow, to maximal flow, by opening and closing the valve (analogous to activating and inhibiting an enzyme). Your choices in the other scenario, filling the pool from a lake, are not so great. It would be hard to fill the water to the desired level (especially if it was an above ground pool). It would be hard to regulate the flow.
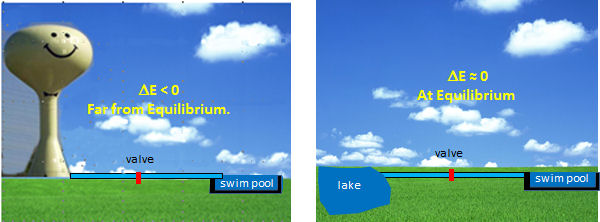
By analogy, the best candidates for regulation are those enzymes whose reactions are thermodynamically favored (not at equilibrium) but which can be controlled by the mechanisms discussed in the previous section.
Which reactions are commonly not at equilibrium (i.e. DG<0 and usually also DG0 <0 if the ratio of products to reactants is not too high)? The answer is those that have reactants that are thermodynamically unstable compared to their reaction products. There are several types of reactions that often fit these criteria:
Hydrolysis (or similar reactions) of anhydride or analogous motifs: The figure below shows molecules with similar "anhydride" motifs and the DG0 for hydrolysis of the molecules. Those with more negative DG0 values can transfer their phosphate group to ADP to make ATP, which is necessary to drive unfavorable biological reactions. Metabolic reactions that involve hydrolysis (or other type of transfer reaction of these groups) usually proceed with a negative DG0 and DG, making them prime candidates for pathway regulation. Many textbooks label these types of molecules as having "high energy" bonds. This is confusing to many student as bonds between atoms lower the energy compare to when the atoms are not bonded. It takes energy to break the "high" energy phosphoanhydride covalent bond. What make hydrolysis of the molecules below so exergonic is that more energy is released on bond formation within the new products than was required to break the bonds in the reactants. In addition, other effects such as preferential hydration of the products, lower charge density in the products, and less competing resonances in the products all contribute to the thermodynamically favorable hydrolysis of the reactants.
Figure: Thermodynamically Unstable Molecules (compared to their reaction products)
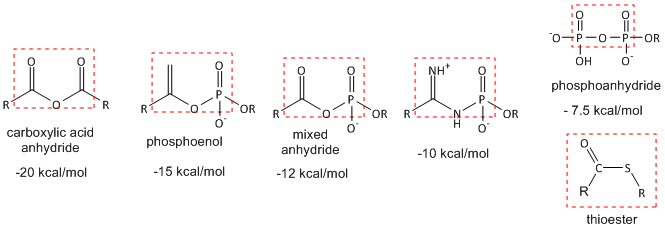
Thioesters (such as Acetyl-SCoA) are also included since that have the same negative DG0 of hydrolysis as ATP, even though the lack an "anhydride" motif. Thioesters are destabilized compared to their hydrolysis products and in comparison to esters made with alcohol since the C-S bond is weaker. Why?
Redox reactions: Everyone knows that redox reactions are thermodynamically favored if the oxidizing agent deployed is strong enough. The oxidation reactions of hydrocarbons, sugars, and fats by dioxygen are clearly exergonic (we do call these combustion reactions after all). What about redox reactions with less powerful oxidants? NAD+ is used frequently as a biological oxidizing agent. Are all these reactions as favored as combustion? Hardly so. Remember that in every redox reaction, an oxidizing and reducing agent react to form another oxidizing and reducing agent. Consider the following reaction:
Pyruvate + NADH --> Lactate + NAD+.
This reaction can go either way and is reversible. In the above
form, it is written in the favored direction in aeroboic metabolism when
both Pyr and NADH level are high. Although the DG0
actually favors the oxidation of lactate, given the high concentration of
Pyr and NADH, the reaction is driven in the opposite direction and
proceeds as shown. To determine if a redox reaction is favored and
likely to occur, and possibly be regulated, the DG0 for a redox
reaction should be calculated from standard reduction potentials, using the
formula
DG0 = -nFE0.
B. Regulation of enzymes catalyzing committed steps in pathways:
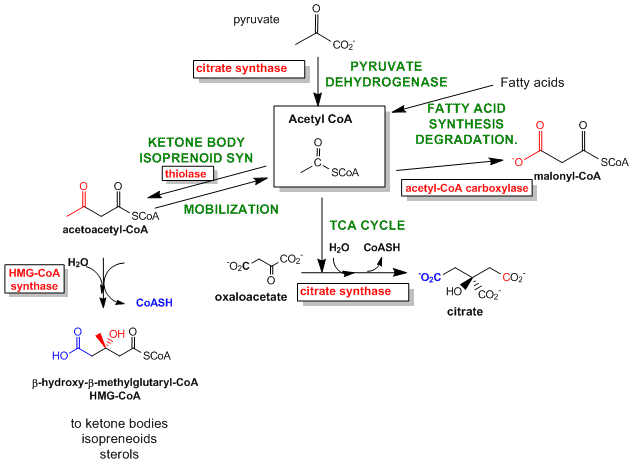
The best enzymes to regulate are those that catalyze the first committed step in the reaction pathway. The committed step proceeds with a DG < 0 and is essentially irreversible. These reactions often occur from key metabolic intermediates that are immediately before or proximal to branches in reaction pathways. Two examples are shown below.
Figure: Reactions for Glucose-6-phosphate
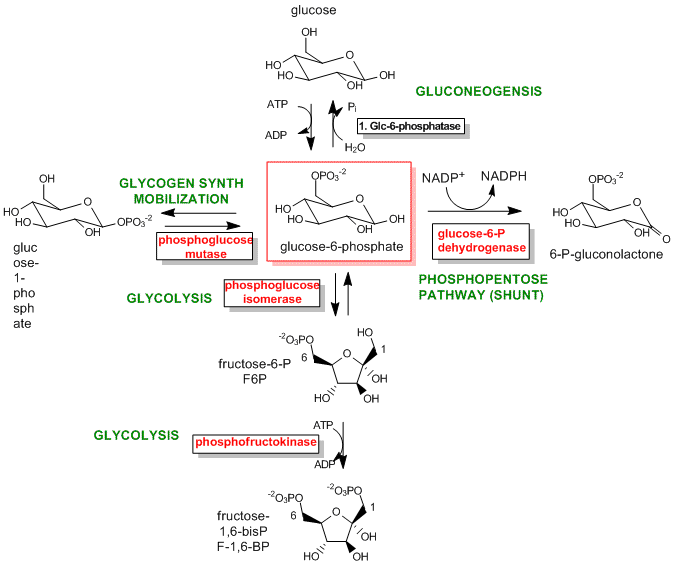
Figure: Reactions for Acetyl-CoA
Navigation:
Navigation
| Reactivity Index | MP0. Metabolic Pathways - Main Menu |
| Structure & Reactivity Web Materials | Biochemistry Online: Table of Contents |

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.