Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 7 - CATALYSIS
E: RIBOZYMES and the RNA World
BIOCHEMISTRY - DR. JAKUBOWSKI
06/10/2014
|
Learning Goals/Objectives for Chapter 7E: After class and this reading, students will be able to
|
"A foolish consistency is the hobgoblin of little minds...." Ralph Waldo Emerson
E1. Ribozymes
Any molecule that displays any of the catalytic motifs seen in the earlier chapters (general acid/base catalysis, electrostatic catalysis, nucleophilic catalysis, intramolecular catalysis, transition state stabilization) can be a catalyst. So far we have examined only protein catalysts. These can fold to form unique 3D structures which can have active sites with appropriate functional groups or nonprotein "cofactors" (metal ions, vitamin derivatives) that participate in catalysis. There is nothing special about the ability of proteins to do this. It is now known that RNA, which can form complicated secondary and tertiary structures as seen in the 3D image of the ribozyme from Tetrahymena thermophila, can as well.
Figure: ribozyme from Tetrahymena thermophila,
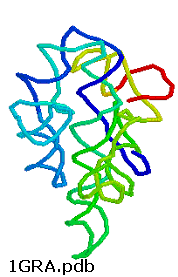
RNA molecules that act as enzymes are called ribozymes. This property of some RNA's was discovered by Sidney Altman and Thomas Czech, who were awarded the Nobel Prize in Chemistry in 1989. In contrast to protein enzymes which are true catalysts in that they are used over again, this is an example of a single use ribozyme. Other ribozymes are true catalysts and can carry out RNA slicing by transesterification (splicesome) and peptidyl transfer (in ribosomes). The mechanisms of catalysis of the hepatitis delta virus ribozyme include general acid/base catalysis.
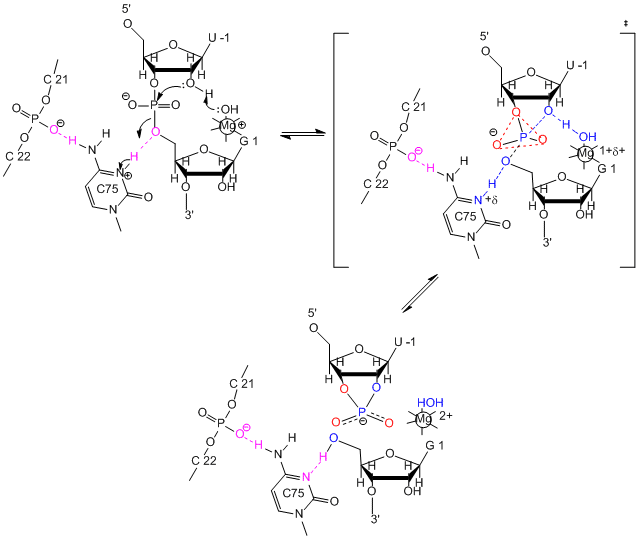
Figure: mechanisms of catalysis
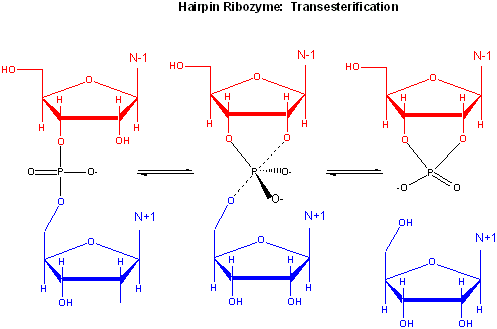
The hairpin ribozyme from satellite RNAs of plant viruses is 50 nucleotides long, and can cleave itself internally, or , in a truncated form, can cleave other RNA strands in a transesterification reaction. The structure consists of two domains, stem A required for binding (self or other RNA molecules) and stem B, required for catalysis. Self-cleavage in the hairpin ribozyme occurs in stem A between an A and G bases (which are splayed apart) when the 2' OH on the A attacks the phosphorous in the phosphodiester bond connecting A and G to form an pentavalent intermediate.
Figure: Self-cleavage in the hairpin ribozyme
Rupert & Ferr�-D'Amar� (2001) solved the crystal structure of a hairpin ribozyme with a non-cleavable substrate analog containing a in which a 2'-OCH3 was substituted for the nucleophilic 2'-OH group. See the applet below.
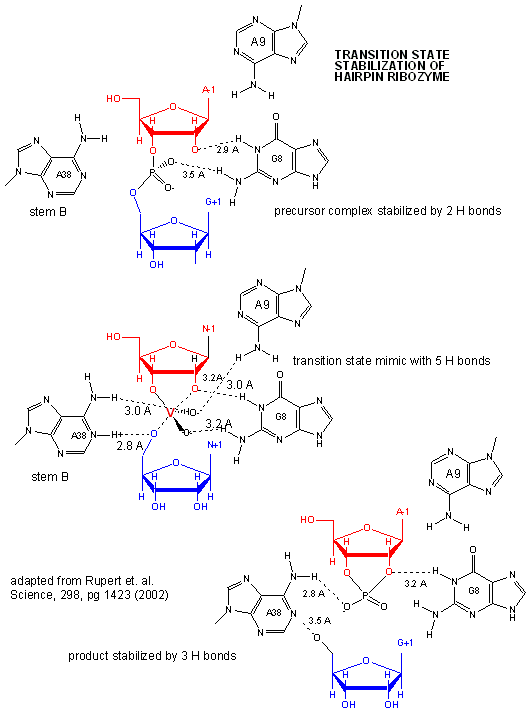
A recent study by Rupert et al. (2002) shows that A38 in Stem B appears to be able to interact with the products (the cleaved A now in the form of a cyclic phosphodiester with itself) and the departing G, and also with a transition state pentavalent analog of the sessile A-G bond in which the phosphodiester linking A and G in the substrate is replaced with a pentavalent vanadate bridge between A and G. However, A 38 does not appear to react with the sessile A -G groups in the normal substrate, indicating that the main mechanism used by this ribozyme is transition state binding. Since RNA molecules have fewer groups available for acid/base and electrostatic catalysis (compared to protein enzymes), ribozymes, presumably the earliest type of biological catalyst, probably make more use of transition state binding as their predominant mode of catalytic activity.
Figure: Active Site of Hairpin Ribozyme: Transition State Binding
Recently, the crystal structure of a purple bacterium group I self-splicing intron (which catalyze the removal of itself) interacting with both exons in a state prior to their ligation was determined (Adams, P. et al.). The structure shows both exons in close proximity. Nucleophilic attack of the 3'OH of the 5' exon on a distorted phosphate at the intron-3'-exon junction. Two metal ions reside on either side of the labile phosphodiester bond at the intron-3'exon junction, and are held in place by 6 phosphates.
A novel use of ribozymes was recently reported by Winkler et. al. They discovered that the 3' end of the mRNA of the gene glmS (from Gram-positive bacteria) which encodes an amidotransferase (catalyzing the formation of glucosamine-6-phosphate from glutamine and fructose-6-phosphate) is a ribozyme. A glucosamine-6-phosphate binding site in the ribozyme (3' end of the mRNA) binds this sugar, inducing autocleavage of the ribozyme. This inhibits, by an uncertain mechanism, the formation of the amidotransferase from the remaining part of the mRNA. This mechanism of regulation of gene expression through ribozyme activity might prove to be common.
In order for RNA molecules to have acted as both catalysts and carriers of genetic information in the original RNA world, RNA must have the potential to self replicate. Shechner et al. looked at the core structure of a class I ligase ribozyme able to polymerize RNA. This ligase was synthesized and enhanced through The tripod structure allows more RNA solvent interaction then often found in ribozymes. Several common structural motifs were also found to be present including the GNRA-triloop, uridine-turn and the frameshifting pseudoknot motifs. GNRA-triloop and uridine-turn motifs are short thermodynamically stable segments that cap the end of the helical regions. Varieties of these are found in many RNA structures although they don’t seem to be necessary for activity. The frameshifting pseudoknot motif is almost identical in structure to small viral ribosomal frameshifting pseudoknots and is adjacent to a new motif named “A-minor triad”. The A-minor triad is responsible for the coordination with Mg2+, although only when inserted in-between certain sequences. Triphosphoguanosine (called G1) at the 5’ terminus of the ribozyme acts as the electrophile for the RNA ligation reaction. Other segments of the ribozyme, such as the J1/2, have highly specific contacts which orient G1 and allow the RNA to fold more efficiently. Two residues, cytosine 12 (C12) and uracil 48 (U48) bind to Mg2+. Other sections of the RNA also promote specificity to assist in the replication process. A model for catalysis and the transition state of the ribozyme polymerase is similar to that for protein RNA polymerases, as shown in the figure below
Figure: Comparison of Transition State Models of Ribozyme and Protein RNA Polymerases (after Schechner et al)
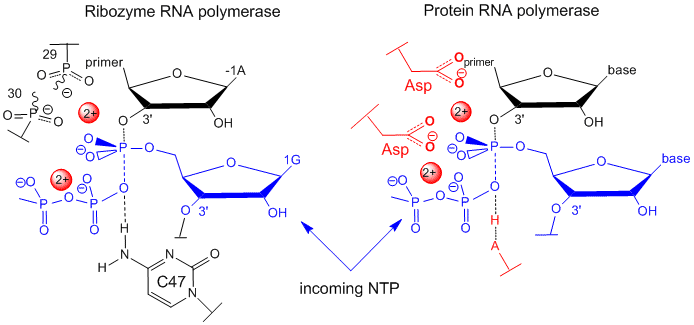
A divalent Mg2+ in the active site of the ribozyme enhances the nuclophilicity of the 3-OH on the primer, which attached the the terminal phosphate of the G(1)TP substrate to form a pentavalent intermediate. The Mg cation is stabilized by oxygens on P 29 and 30 of the ribozyme. The Mg ion also stabilizes the developing charge in the transition state and in the charge in the intermediate. Stabilization of analogous divalent cations in the protein polymerase occurs through Asp side changes in the protein.
- Hairpin Ribozyme Add 1HP6.pdb
![]() Jmol:
Updated Self-splicing Group I intron with both exons
Jmol14 (Java) |
JSMol (HTML5)
Jmol:
Updated Self-splicing Group I intron with both exons
Jmol14 (Java) |
JSMol (HTML5)
![]() Jmol:
Updated L1 Ligase Ribozyme
Jmol14 (Java) |
JSMol (HTML5) Not done; Fix
Jmol:
Updated L1 Ligase Ribozyme
Jmol14 (Java) |
JSMol (HTML5) Not done; Fix
-
 3D
Structure of Ribozyme with Active Site - From the Howard Hughes
Medical Institute.
3D
Structure of Ribozyme with Active Site - From the Howard Hughes
Medical Institute.
E2. The Biggest Ribozyme - The Ribosome
Protein synthesis from a mRNA template occurs on a ribosome, a nanomachine composed of proteins and ribosomal RNAs (rRNA). The ribosome is composed of two very large structural units. The smaller unit (termed 30S and 40S in bacteria and eukaryotes, respectively) coordinates the correct base pairing of the triplet codon on the mRNA with another small adapter RNA, transfer or tRNA, that brings a covalently connected amino acid to the site. Peptide bond formation occurs when another tRNA-amino acid molecule binds to an adjacent codon on mRNA. The tRNA has a cloverleaf tertiary structure with some intrastranded H-bonded secondary structure. The last three nucleotides at the 3' end of the tRNA are CpCpA. The amino acid is esterified to the terminal 3'OH of the terminal A by a protein enzyme, aminoacyl-tRNA synthetase.
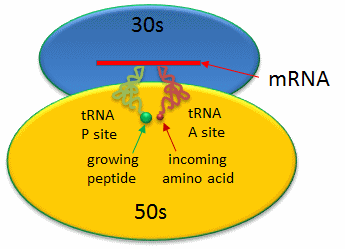
Covalent amide bond formation between the second amino acid to the first, forming a dipeptide, occurs at the peptidyl transferase center, located on the larger ribosomal subunit (50S and 60S in bacteria and eukaryotes, respectively). The ribosome ratchets down the mRNA so the dipeptide-tRNA is now at the the P or Peptide site, awaiting a new tRNA-amino acid at the A or Amino site. The figure below shows a schematic of the ribosome with bound mRNA on the 30S subunit and tRNAs covalently attached to amino acid (or the growing peptide) at the A and P site, respectively.
Figure: Prokaryotic Ribosme - P and A sites
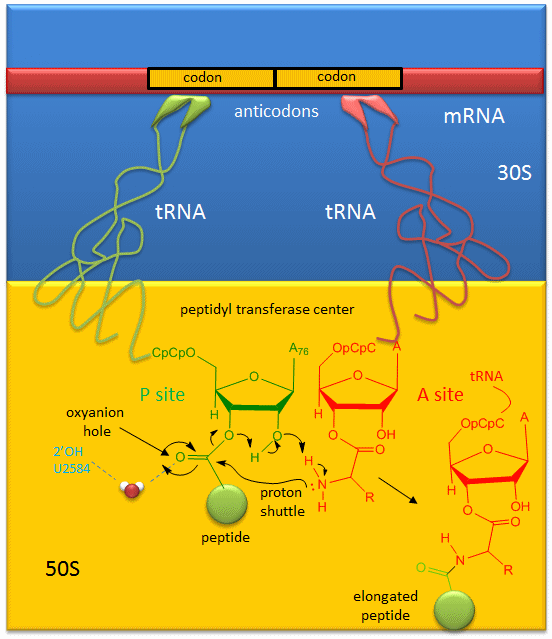
A likely mechanism (derived from crystal structures with bound substrates and transition state analogs) for the formation of the amide bond between a growing peptide on the P-site tRNA and the amino acid on the A-site tRNA is shown below. Catalysis does not involve any of the ribosomal proteins (not shown) since none is close enough to the peptidyl transferase center to provide amino acids that could participate in general acid/base catalysis, for example. Hence the rRNA must acts as the enzyme (i.e. it is a ribozyme). Initially it was thought that a proximal adenosine with a perturbed pKa could, at physiological pH, be protonated/deprotonated and hence act as a general acid/base in the reaction. However, none was found. The most likely mechanism to stabilize the oxyanion transition state at the electrophilic carbon attack site is precisely located water, which is positioned at the oxyanion hole by H-bonds to uracil 2584 on the rRNA. The cleavage mechanism involves the concerted proton shuffle shown below. In this mechanism, the substrate (Peptide-tRNA) assists its own cleavage in that the 2'OH is in position to initiate the protein shuttle mechanism. (A similar mechanism might occur to facilitate hydrolysis of the fully elongated protein from the P-site tRNA.) Of course all of this requires perfect positioning of the substrates and isn't that what enzymes do best? The main mechanisms for catalysis of peptide bond formation by the ribosome (as a ribozyme) are intramolecular catalysis and transition state stabilization by the appropriately positioned water molecule.
Figure: Mechanism Peptide Bond Formation by the Ribosome
The crystal structure of the eukaryotic ribosome has recently been published (Ben-Shem et al). It is significantly larger (40%) with mass of around 3x106 Daltons. The 40S subunit has one rRNA chain (18) and 33 associated proteins, while the larger 60S subunit has 3 rRNA chains (25S, 5.8S and 5S) and 46 associated proteins. The larger size of the eukaryotic ribosome facilitates more interactions with cellular proteins and greater regulation of cellular events. The Jmol structure of a bacterial 70S ribosome showing mRNA and tRNA interactions is shown below.
![]() Jmol:
Updated 70 S Ribosome from Thermus Thermophilus showing mRNA
and tRNA interactions Jmol14 (Java) | JSMol
(HTML5) Not done; Fix
Jmol:
Updated 70 S Ribosome from Thermus Thermophilus showing mRNA
and tRNA interactions Jmol14 (Java) | JSMol
(HTML5) Not done; Fix
E3. The RNA World
Given that RNA expresses catalytic activities and can carry genetic information (some viruses have ds and ss RNA as their genome), it has been suggested that early life might have been based on RNA. DNA would evolve later as a more secure carrier of genetic information. An inspection of chemical properties of DNA, RNA, and proteins shows them to have attributes needed for their expressed function. Let's examine each for structural features that might be important for function.
a. Why does DNA lack a 2' OH group (found in RNA), which has been replaced with a hydrogen? This required the evolutionary creation of a new enzyme, ribonucleotide reductase, to catalyze the replacement of the OH in a ribonucleotide monomer to form the deoxyribonucleotide form. One possible explanation if offered in the figure below. DNA, the main carrier of genetic information, must be an extremely stable molecules. An OH present on C'2 could act as a nucleophile and attack the proximal P in the phosphodiester bond, leading to a nucleophilic substitution reaction and potential cleavage of the link. RNA, an intermediary molecule, whose concentration (at least as mRNA) should rise and fall based on the need for a potential transcript, should be more labile to such hydrolysis.
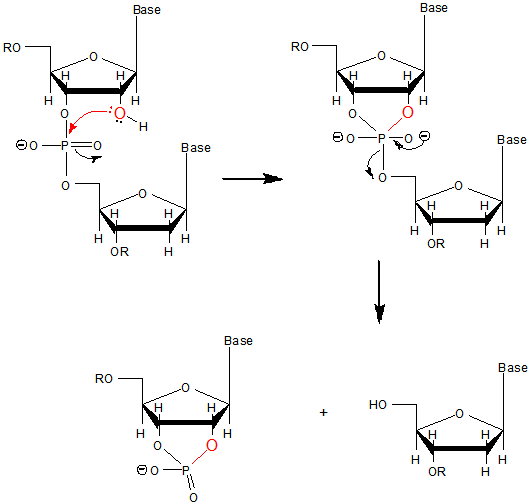
b. Why do both DNA and RNA contain a phosphodiester link between adjacent monomers instead of more "traditional" links such as carboxylic acid esters, amides, or anhydrides? One possible explanation is given below. Nucleophilic attack on the sp3 hybridized P in a phosphodiester is much more difficult than for a more open sp2 hybridized carboxylic acid derivative. In addition, the negative charge on the O in the phosphodiester link would decrease the likelihood of a nucleophilic attack. The negative charges on both strands in ds-DNA probably helps keep the strands separated allowing the traditional base pairing and double stranded helical structure observed.
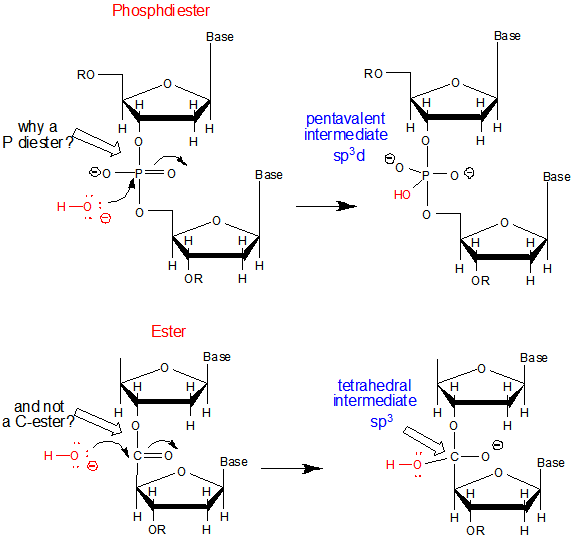
c. Why is DNA found as a repetitive double-stranded helix but RNA is usually found as a single stranded molecule which can form complicated tertiary structures with some ds-RNA motifs?
Another reason for the absence of the 2' OH in DNA is that it allows the deoxyribose ring in DNA to pucker in just the right way to sterically allow extended ds-DNA helices (B type). The pucker in deoxyribose and ribose can be visualized by visualizing a single plane in the sugar ring defined by the ring atoms C1', O and C4'. If a ring atom is pointing in the same direction as the C4'-C5' bond, the ring atom is defined as endo. If it is pointing in the opposite direction, it is defined as exo (see Jmol below). In the most common form of double-stranded DNA, B-DNA, which is the iconic extended double helix you know so well, C2' is in the endo form. It can also adopt the C3' endo form, leading to the formation of another less common helix, more open ds-A helix. In contrast, steric interference prevents ribose in RNA from adopting the 2'endo conformation, and allows only the 3'endo form, precluding the occurrences of extended ds-B-RNA helices but allowing more open, A-type helices.
![]() Jmol:
Puckering in ribose and deoxyribose
Jmol:
Puckering in ribose and deoxyribose
![]() Jsmol:
Jsmol:
![]() Comparison
of ds DNA forms
Comparison
of ds DNA forms
![]() Jmol:
Comparison of ds DNA and RNA
Jmol:
Comparison of ds DNA and RNA
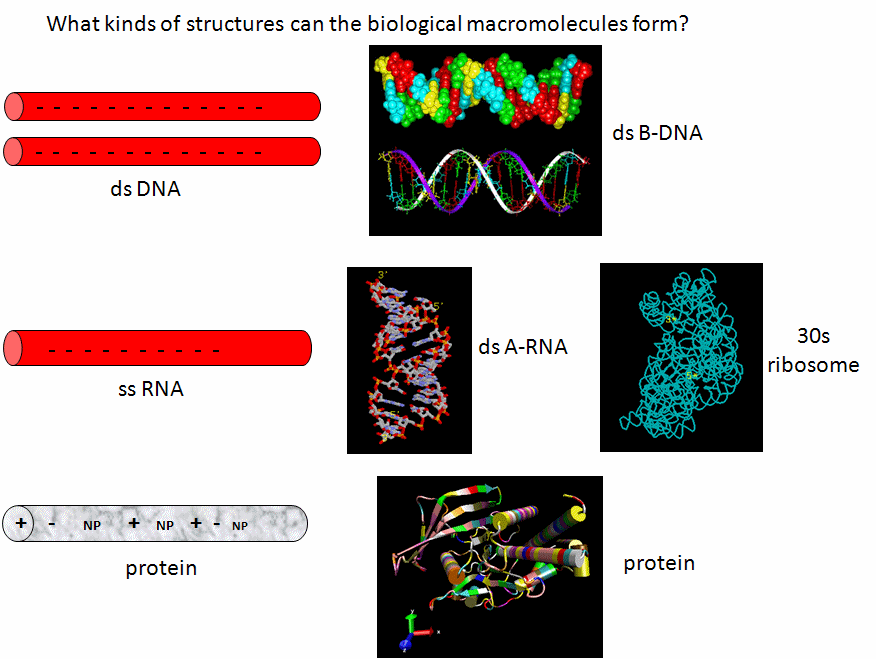
Now lets review the kinds of structure adopted by the 3 major macromolecules, DNA, RNA and proteins. DNA predominately adopts the classic ds-BDNA structure, although this structure is wound around nucleosomes and "supercoiled" in cells since it must be packed into the nucleus. This extended helical form arise in part from the significant electrostatic repulsions of two strands of this polyanions (even in the presence of counterions). Given its high charge density, it is not surprising that it is complexed with positive proteins and does not adopt complex tertiary structures. RNA, on the other hand, can not form long B-type double-stranded helices (due to steric constraints of the 2'OH and the resulting 3'endo ribose pucker). Since it doesn't have the same charge density as the double-stranded DNA, it can adopt complex tertiary conformations (albeit with significant counterion binding to stabilize the structure) and in doing so can form regions of secondary structure (ds-A RNA) in the form of stem/hairpin forms. Proteins, with its combination of polar charged, polar uncharged, and nonpolar side chains have little electrostatic hindrance in the adoption of secondary and tertiary structures. That RNA and proteins can both adopt tertiary structures with potential binding and catalytic sites makes them ideal catalysts for chemical reactions. RNA, given its 4 nucleotide motif can clearly also carry genetic information, making it an ideal candidate for the first evolved macromolecules enabling the development of life. Proteins with a great abundance of organic functionalities would eventually supplant RNA as a better choice for life's catalyst. DNA, with its greater stability, would supplant RNA as the choice for the main carrier of genetic information.
E4. Links and References
Ben-Shem, A. et al. Crystal Structure of the Eukaryotic Ribosome. Science 330, 1203 (2010)
Schmeing, T. and Ramakrishan, V. What recent ribosome structures have revealed about the mechanism of translation. Nature 461, 1234 (2009)
Rodina, M. et al. How ribosomes make peptide bonds. TIBS 32, 20 (2007)
M. Simonović, T.A. Steitz, A structural view on the mechanism of the ribosome-catalyzed peptide bond formation,Biochim. Biophys. Acta (2009), doi:10.1016/j.bbagrm.2009.06.006

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.