Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 1 - LIPID STRUCTURE
A: Lipid Structure
BIOCHEMISTRY - DR. JAKUBOWSKI
2/06/16
Learning Goals/Objectives for Chapter 1A: After class and this reading, students will be able to
|
Lipids
Lipids are small biological molecules which are soluble in organic solvents, such as chloroform/methanol, and are sparingly soluble in aqueous solutions. They can be classified in a variety of ways. In one categorization, they can be divided into two majors classes, saponifiable and nonsaponifiable lipids, based on their reactivity with strong bases. Saponifiable lipids contain long chain carboxylic (of fatty) acids, that are linked to an alcoholic functional group through an ester linkage. These fatty acids are released on based catalyzed ester hydrolysis. The nonsaponifiable classes include the "fat-soluble" vitamins (A, E) and cholesterol. Lipids are often distinguished from another commonly used word, fats. Some define fats as lipids that contain fatty acids that are esterified to glycerol. I will use the lipid and fat synonymously.
Figure: Examples saponifiable and nonsaponifiable lipids
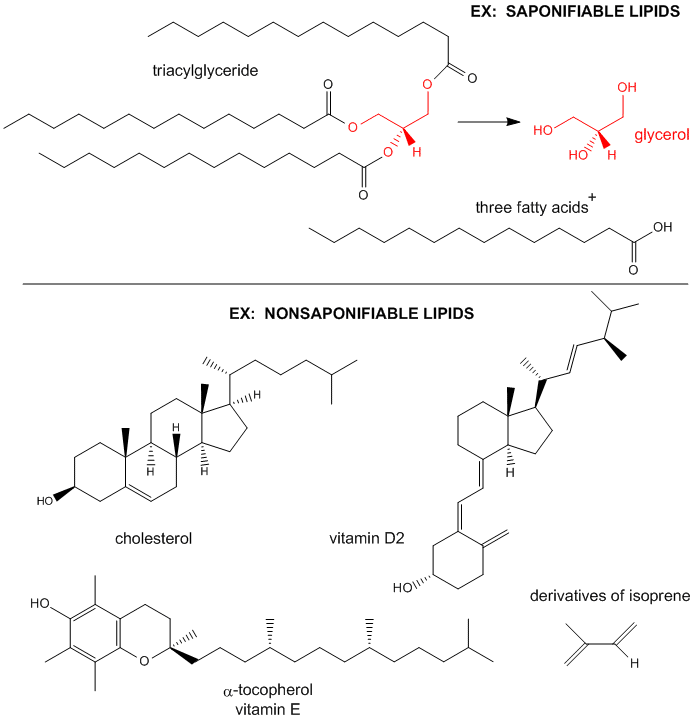
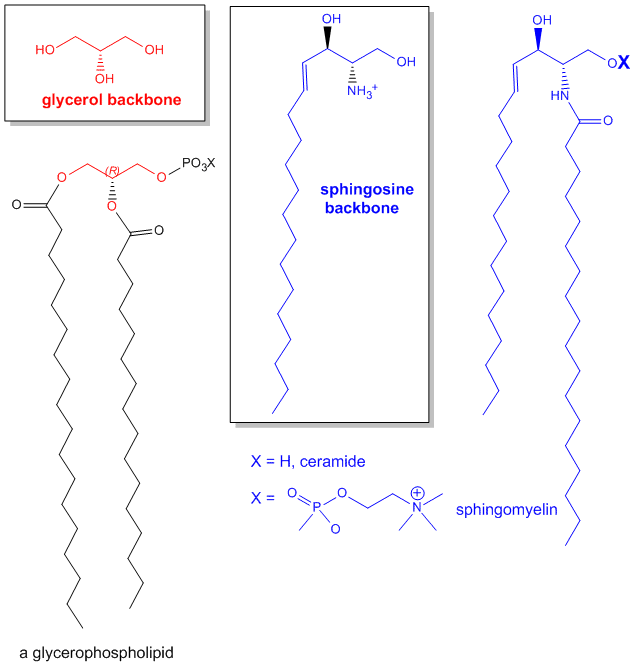
The major saponifiable lipids are triacylglycerides, glycerophospholipids, and the sphingolipids. The first two use glycerol as the backbone. Triacylglycerides have three fatty acids esterified to the three OHs on glycerol. Glycerophospholipids have two fatty acids esterified at carbons 1 and 2, and a phospho-X groups esterifed at C3. Sphingosine, the backbone for sphingolipids, has a long alkyl group connected at C1 and a free amine at C2, as a backbone. In sphingolipids, a fatty acid is attached through an amide link at C2, and a H or esterified phospho-X group is found at C3. A general diagrams showing the difference in these structures is shown below.
Figure: Comparison of lipids with glycerol and sphingosine as backbones
The simple classification of lipids based on their reactivity towards bases belies the complexity of possible lipid structures as over 1000 different lipids are found in eukaryotic cells. This complexity has led to the development of a comprehensive classification system for lipids. In this system, lipids are given a very detailed as well as all-encompassing definition: "hydrophobic or amphipathic small molecules that may originate entirely or in part by carbanion-based condensations of thioesters (fatty acyl, glycerolipids, glycerophospholipids, sphingolipds, saccharolipds and polyketides) and/or by carbocation-based condensations of isoprene units (prenol lipids and sterol lipids)."
Using this new nomenclature, lipids can be broken into eight different categories, as shown in the table below and at LIPID MAPS.
Table: Classification of Lipids Based on International Classification and Nomenclature Committee
| Category | Abbreviation | Example Structure |
| Fatty Acids | FA |
 |
| Glycerolipids | GL |
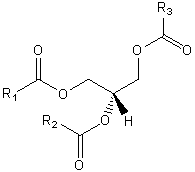 |
| Glycerophospholipids | GP |
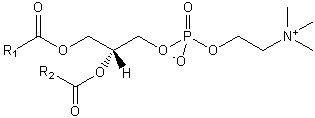 |
| Sphingolipids | SP |
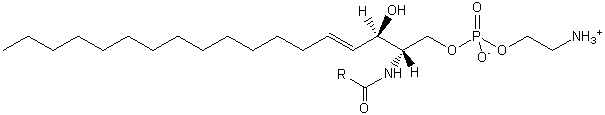 |
| Sterol Lipids | ST |
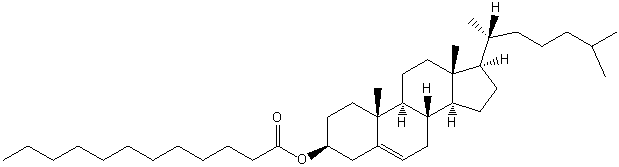 |
| Prenol Lipids | PR |
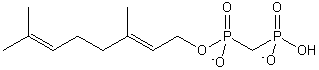 |
| Saccharolipids (contain sugar) |
SL |
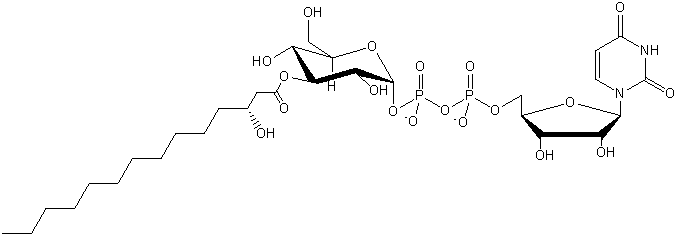 |
| Polyketides | PK |
 |
Properties of Lipids
The structure of lipids determines their function. For example, the very insoluble triacylglycerides are used as the predominant storage form of chemical energy in the body. In contrast to polysaccharides such as glycogen (a polymer of glucose), the Cs in the acyl-chains of the triacylglyceride are in a highly reduced state. The main source of energy to drive not only our bodies but also our society is obtained through oxidizing carbon-based molecules to carbon dioxide and water, in a reaction which is highly exergonic and exothermic. Sugars are already part way down the free energy spectrum since each carbon is partially oxidized. 9 kcal/mol can be derived from the complete oxidation of fats, in contrast to 4.5 kcal/mol from that of proteins or carbohydrates. In addition, glycogen is highly hydrated. For every 1 g of glycogen, 2 grams of water is H-bonded to it. Hence it would take 3 times more weight to store the equivalent amount of energy in carbohydrates as is stored in triacylglyceride, which are stored in anhydrous lipid "drops" within cells . The rest of this unit on lipids will focus not on triacylglycerides, whose main function is energy storage, but on fatty acids and phospholipids, and the structures they form in aqueous solution.
The structure of fatty acids and phospholipids show them to amphiphilic - i.e. they have both hydrophobic and hydrophilic domains. Fatty acids can be represented in "cartoon-form" as single chain amphiphiles with a circular polar head group and a single acyl non-polar tail extending from the head. Likewise, phospholipids can be shown as double chain amphiphiles. Even cholesterol can be represented this way, with its single OH group as the polar head, and the rigid 4 member rings as the hydrophobic “tail”. Even through there are a very large number of fatty acids which can be esterified to C1 and C2 of phospholipids and a variety of P-X groups at C3, making the phospholipids and fatty acids extremely heterogeneous groups of molecules, their role in biological structures can be understood to a first approximation by modeling them either as single or double chain amphiphiles. In addition, they, in contrast to carbohydrates, amino acids, and nucleotides, do not form covalent polymers. Hence we will start our studies of biological molecules with simple lipids (fatty acids, glycerophospholipids and sphingolipids) and then apply our understanding of lipids to the more complex systems of biological polymers. We will see that glycerophospholipids and sphingolipids are essential components of membrane structure. Cholesterol is also found in membranes and is a precursor of steroid hormones.
Fatty Acids
Fatty acids can be saturated (contain no double bonds in the acyl chain), or unsaturated (with either one -monounsaturated - or multiple - polyunsaturated - double bond(s)) . The table below gives the names, in a variety of formats, of common fatty acids.
Table: Names and structures of the most common fatty acids
COMMON BIOLOGICAL SATURATED FATTY ACIDS
|
Symbol |
common name |
systematic name |
structure |
mp(C) |
|
12:0 |
Lauric acid |
dodecanoic acid |
CH3(CH2)10COOH |
44.2 |
|
14:0 |
Myristic acid |
tetradecanoic acid |
CH3(CH2)12COOH |
52 |
|
16:0 |
Palmitic acid |
Hexadecanoic acid |
CH3(CH2)14COOH |
63.1 |
|
18:0 |
Stearic acid |
Octadecanoic acid |
CH3(CH2)16COOH |
69.6 |
|
20:0 |
Arachidic aicd |
Eicosanoic acid |
CH3(CH2)18COOH |
75.4 |
COMMON BIOLOGICAL UNSATURATED FATTY ACIDS
|
Symbol |
common name |
systematic name |
structure |
mp(C) |
|
16:1D9 |
Palmitoleic acid |
Hexadecenoic acid |
CH3(CH2)5CH=CH-(CH2)7COOH |
-0.5 |
|
18:1D9 |
Oleic acid |
9-Octadecenoic acid |
CH3(CH2)7CH=CH-(CH2)7COOH |
13.4 |
|
18:2D9,12 |
Linoleic acid |
9,12 -Octadecadienoic acid |
CH3(CH2)4(CH=CHCH2)2(CH2)6COOH |
-9 |
|
18:3D9,12,15 |
a-Linolenic acid |
9,12,15 -Octadecatrienoic acid |
CH3CH2(CH=CHCH2)3(CH2)6COOH |
-17 |
|
20:4D5,8,11,14 |
arachidonic acid |
5,8,11,14- Eicosatetraenoic acid |
CH3(CH2)4(CH=CHCH2)4(CH2)2COOH |
-49 |
|
20:5D5,8,11,14,17 |
EPA |
5,8,11,14,17-Eicosapentaenoic- acid |
CH3CH2(CH=CHCH2)5(CH2)2COOH |
-54 |
|
22:6 D4,7,10,13,16,19 |
DHA |
Docosohexaenoic acid |
22:6w3 |
|
% FATTY ACIDS IN VARIOUS FATS
|
FAT |
<16:0 |
16:1 |
18:0 |
18:1 |
18:2 |
18:3 |
20:0 |
22:1 |
22:2 |
. |
|
Coco-nut |
87 |
. |
3 |
7 |
2 |
. |
. |
. |
. |
. |
|
Canola |
3 |
. |
|
11 |
13 |
10 |
. |
7 |
50 |
2 |
|
Olive Oil |
11 |
. |
4 |
71 |
11 |
1 |
. |
. |
. |
. |
|
Butter-fat |
50 |
4 |
12 |
26 |
4 |
1 |
2 |
. |
. |
. |
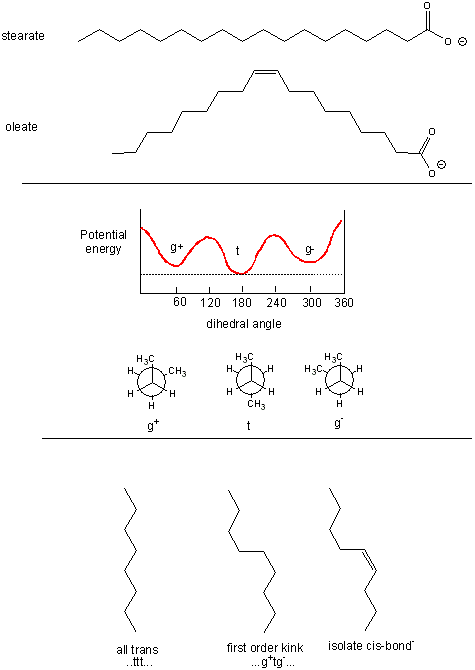
The figure below shows the relative conformations of saturated and unsaturated fatty acids, and in comparison, the conformations and potential energy graph for n-butane, which should provide insight into conformational changes in the nonpolar tail of fatty acids arising from rotation around C-C single bonds. We will explore this diagram a bit latter.
Figure: Conformations of fatty acids and n-butane
![]()
![]() Jmol:
conformations of ethane |
conformations of propane |
butane: the gauche conformation
Jmol:
conformations of ethane |
conformations of propane |
butane: the gauche conformation
Fatty acids can be named in many ways.
- symbolic name: given as x:y (D a,b,c) where x is the number of C’s in the chain, y is the number of double bonds, and a, b, and c are the positions of the start of the double bonds counting from C1 - the carboxyl C. Saturated fatty acids contain no C-C double bonds. Monounsaturated fatty acids contain 1 C=C while polyunsaturated fatty acids contain more than 1 C=C. Double bonds are usual cis.
- systematic name using IUPAC nomenclature. The systematic name gives the number of Cs (e.g. hexadecanoic acid for 16:0). If the fatty acid is unsaturated, the base name reflects the number of double bonds (e.g. octadecenoic acid for 18:1 D 9 and octadecatrienoic acid for 18:3D 9,12,15).
- common name: (e.g. oleic acid, which is found in high concentration in olive oil)
You should know the common name, systematic name, and symbolic representations for these saturated fatty:
- lauric acid, dodecanoic acid, 12:0
- palmitic acid, hexadecanoic acid, 16:0
- stearic acid, octadecanic acid, 18:0.
Learn the following unsaturated fatty acids -
- oleic acid, octadecenoic acid, 18:1 D 9
- linoleic acid, octadecadienoic acid, 18:2 D 9,12
- a-linolenic acid, octadecatrienoic acid, 18:3 D 9,12,15 (n-3)
- arachidonic acid, eicosatetraenoic acid, 20:4 D 5,8,11,14 (n-6)
- eicosapentenoic acid (EPA), 20:5 D 5,8,11,14,17 (n-3) Note: sometimes written as eicosapentaenoic
- docosahexenoic acid (DHA) 22:6 D4,7,10,13,16,19 (n-3) Note: sometimes written as docosahexaenoic
There is an alternative to the symbolic representation of fatty acids, in which the Cs are numbered from the distal end (the n or w end) of the acyl chain (the opposite end from the carboxyl group). Hence 18:3 D 9,12,15 could be written as 18:3 (w -3) or 18:3 (n -3) where the terminal C is numbered one and the first double bond starts at C3. Arachidonic acid is an (w -6) fatty acid while docosahexaenoic acid is an (w -3) fatty acid.
Note that all naturally occurring double bonds are cis (E), with a methylene spacer between double bonds - i.e. the double bonds are not conjugated. For saturated fatty acids, the melting point increases with C chain length, owing to increased likelihood of van der Waals (London or induced dipole) interactions between the overlapping and packed chains. Within chains of the same number of Cs, melting point decreases with increasing number of double bonds, owing to the kinking of the acyl chains, followed by decreased packing and reduced intermolecular forces (IMFs). Fatty acid composition differs in different organisms:
- animals have 5-7% of fatty acids with 20-22 carbons, while fish have 25-30%
- animals have <1% of their fatty acids with 5-6 double bonds, while plants have 5-6% and fish 15-30%
Many studies support the claim the diets high in fish that contain abundant n-3 fatty acids, in particular EPA and DHA, reduce inflammation and cardiovascular disease. n-3 fatty acids are abundant in high oil fish (salmon, tuna, sardines), and lower in cod, flounder, snapper, shark, and tilapia.
The most common polyunsaturated fats (PUFAs) in our diet are the n-3 and n-6 classes. Most abundant in the n-6 class in plant food is linoleic acid (18:2n-6, or 18:2D9,12), while linolenic acid (18:3n-3 or 18:3D9,12,15) is the most abundant in the n-3 class. These fatty acids are essential in that they are biological precursors for other PUFAs. Specifically,
- linoleic acid (18:2n-6, or 18:2D9,12) is a biosynthetic precursor of arachidonic acid (20:4n-6 or 20:4D5,8,11,14)
- linolenic acid (18:3n-3, or 18:3D9,12,15) is a biosynthetic precursor of eicosapentaenoic acid (EPA, 20:5n-3 or 20:5D5,8,11,14,17) and to a much smaller extent, docosahexaenoic acid (DHA, 22:6n-3 or 22:6D4,7,10,13,16,19).
These essential precursor fatty acids are substrates for intracelluar enzymes such as elongases, desaturases, and beta-oxidation type enzymes in the endoplasmic reticulum and another organelle, the peroxisome (involved in oxidative metabolism of straight chain and branched fatty acids, peroxide metabolism, and cholesterol/bile salt synthesis). Animals fed diets high in plant 18:2(n-6) fats accumulate 20:4(n-6) fatty acids in their tissues while those fed diets high in plant 18:3(n-3) accumulate 22:6(n-3. Animals fed diets high in fish oils accumulate 20:5 (EPA) and 22:6 (DHA) at the expense of 20:4(n-6).
Recent work has suggested that contrary to images of early hominids as hunters and scavengers of meat, human brain development might have required the consumption of fish which is highly enriched in arachidonic and docosahexaenoic acids. A large percent of the brain consists of lipids, which are highly enriched in these two fatty acids. These acids are necessary for the proper development of the human brain and in adults, deficiencies in these might contribute to cognitive disorders like ADHD, dementia, and dyslexia. These fatty acids are essential in the diet, and probably could not have been derived in high enough amounts from the eating of brains of other animals. The mechanism for the protective effects of n-3 fatty acids in health will be explored later in the course when we discuss prostaglandins synthesis and signal transduction.
Saturated fatty acids chains can exist in many conformations resulting from free rotation around the C-C bonds of the acyl chains. A quick review of the conformations of n-butane shows that the energetically most favorable conformation is one in which the two CH3 groups attached to the 2 methylene C’s (C2 and C3) are trans to each other, which results in decreased steric strain. Looking at a Neuman projection of n-butane shows the dihedral or torsional angle of this trans conformation to be 180 degrees. When the dihedral angle is 0 degrees, the two terminal CH3 groups are syn to each other, which is the conformation of highest energy. When the angle is 60 (gauche+) or 300 (gauche-) degrees, a higher, local minimum is observed in the energy profile. At a given temperature and moment, a population of molecules of butane would consist of some in the g+ and g- state, with most in the t state. The same applies to fatty acids. To increase the number of chains with g+tg- conformations, for example, the temperature of the system can be increased.
Glycerophospholipid and Sphingolipids
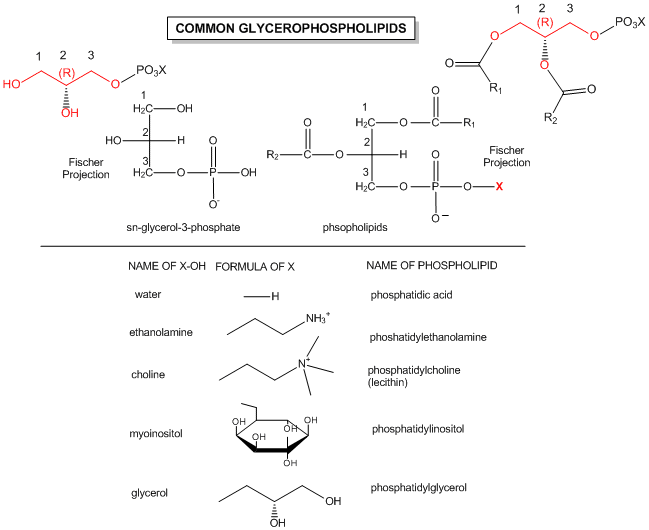
The generic structures of glycerophospholipid is show below, along with the most common glycerophospholipids. Learn the structures of phosphatic acid (PA), phosphatidyethanolamine (PE), phosphatidylcholine (PC) which is often called lechitin, phosphatidylserine (PS) which is often called cephalin, and sphingomylein (shown in an earlier figure).
Figure: Structures of common phospholipids
![]() Updated
di-18:0 PC
Jmol14 (Java) |
JSMol (HTML5)
Updated
di-18:0 PC
Jmol14 (Java) |
JSMol (HTML5)
![]() Updated
Triacylglyceride
Jmol14 (Java) |
JSMol (HTML5)
Updated
Triacylglyceride
Jmol14 (Java) |
JSMol (HTML5)
Triacylglyceride/Phospholipid Stereochemistry
Glycerol is an achiral molecule, since C2 has two identical substituents, -CH2OH. Glycerol in the body can be chemically converted to triacylglycerides and phospholipids (PL) which are chiral, and which exist in one enantiomeric form. How can this be possible if the two CH2OH groups on glycerol are identical? It turns out that even though these groups are stereochemically equivalent, we can differentiate them as follows. Orient glycerol with the OH on C2 pointing to the left. Then replace the OH of C1 with OD, where D is deuterium. Now the two alcohol substituents on C1 and C3 are not identical and the resulting molecule is chiral. By rotating the molecule such that the H on C2 points to the back, and assigning priorities to the other substituents on C2 as follows: OH =1, DOCH2 =2, and CH2OH = 3, it can be seen that the resulting molecule is in the S configuration. Hence we say that C1 is the proS carbon. Likewise, if we replaced the OH on C3 with OD, we will form the R enantiomer. Hence C3 is the proR carbon. This shows that in reality we can differentiate between the two identical CH2OH substituents. We say that glycerol is not chiral, but prochiral. (Think of this as glycerol has the potential to become chiral by modifying one of two identical substituents.)
Figure: Glycerol - A prochiral molecule
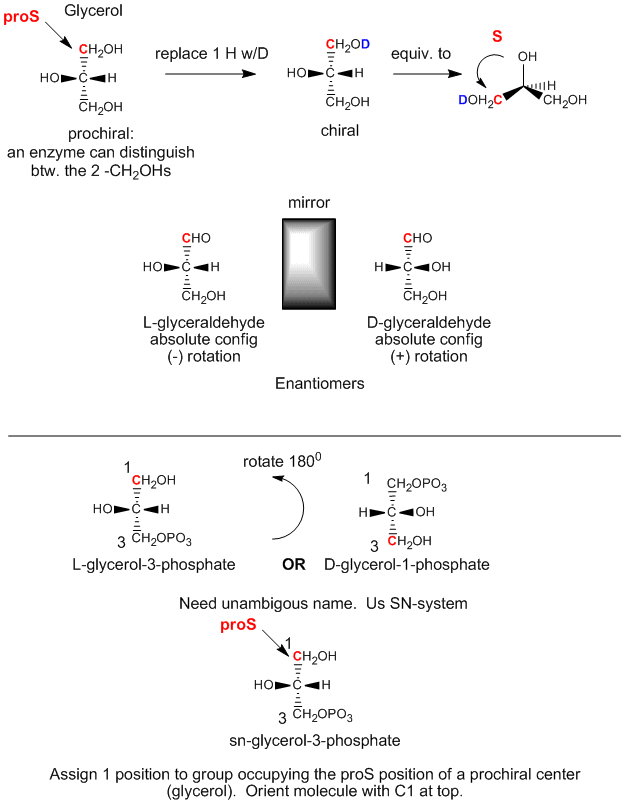
We can relate the configuation of glycerol above, (when OH on C2 is pointing to the left) to the absolute configuration of L-glyceraldehyde, a simple sugar (a polyhydroxyaldehyde or ketone), another 3C glycerol derivative. This molecule is chiral with the OH on C2 (the only chiral carbon) pointing to the left. It is easy to remember that any L sugar has the OH on the last chiral carbon pointing to the left. The enantiomer (mirror image isomer) of L-glyceraldehyde is D-glyeraldehyde, in which the OH on C2 points to the right. Biochemists use L and D for lipid, sugar, and amino acid stereochemistry, instead of the R,S nomenclature you used in organic chemistry. The stereochemical designation of all the sugars, amino acids, and glycerolipids can be determined from the absolute configuration of L- and D-glyceraldehyde.
The first step in the in vivo (in the body) synthesis of chiral derivatives from the achiral glycerol involves the phosphorylation of the OH on C3 by ATP (a phosphoanhydride similar in structure to acetic anhydride, an excellent acetylating agent) to produce the chiral molecule glycerol phosphate. Based on the absolute configuration of L-glyceraldehyde, and using this to draw glycerol (with the OH on C2 pointing to the left), we can see that the phosphorylated molecule can be named L-glycerol-3-phosphate. However, by rotating this molecule 180 degrees, without changing the stereochemistry of the molecule, we don't change the molecule at all, but using the D/L nomenclature above, we would name the rotated molecule as D-glycerol-1-phosphate. We can’t give the same molecule two different names. Hence biochemists have developed the stereospecific numbering system (sn), which assigns the 1-position of a prochiral molecule to the group occupying the proS position. Using this nomenclature, we can see that the chiral molecule described above, glycerol-phosphate, can be unambiguously named as sn-glycerol-3-phosphate. The hydroxyl substituent on the proR carbon was phosphorylated.
Figure: The biological synthesis of triacylglycerides and phosphatidic acid from prochiral glycerol
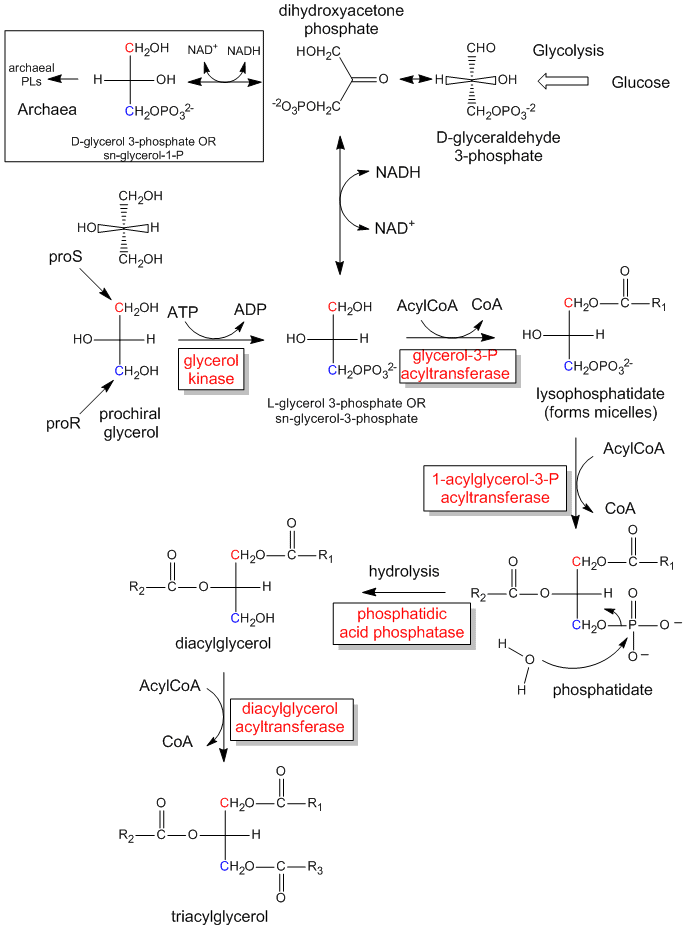
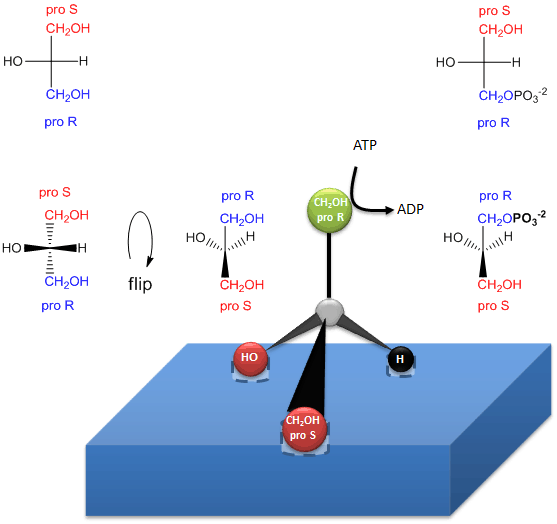
The enzymatic phosphorylation of prochiral glycerol on OH of the proR carbon to form sn-glycerol-3-phosphate is illustrated in the link below. As we were able to differentiate the 2 identical CH2OH substitutents as containing either the proS or proR carbons, so can the enzyme. The enzyme can differentiate identical substituents on a prochiral molecule if the prochiral molecule interacts with the enzyme at three points. Another example of a prochiral reactants/enzyme system involves the oxidation of the prochiral molecule ethanol by the enzyme alcohol dehydrogenase, in which only the proR H of the 2 H’s on C2 is removed. (We will discuss this later.)
Figure: How an enzyme (glycerol kinase)
transfers a PO4 from ATP to the proR CH2OH of glycerol
on
formation of chiral triacylglycerols and phosphatidic acid.
Lipids in Archea, Prokaryotes and Eukaryotes
Do all organisms use the same lipid building blocks to construct bilayers? It turns out they don't. Life can be divided into three separate domains, Bacteria, Archea, and Eukaryota. Studies of sequence similarities of the ribosomal RNA genes from the DNA of these cells show that archea and eukaryota are more closely related than bacteria (also called prokaryotes). Yet there are many similarities between archea and bacteria. Both bacteria and archea are single-celled organisms without nuclei and internal organelles. In the past archea were thought to belong to the prokaryotes. Yet they differ significantly in genetic structure and in their metabolic pathways.
Figure: Phylogenetic Tree of Life
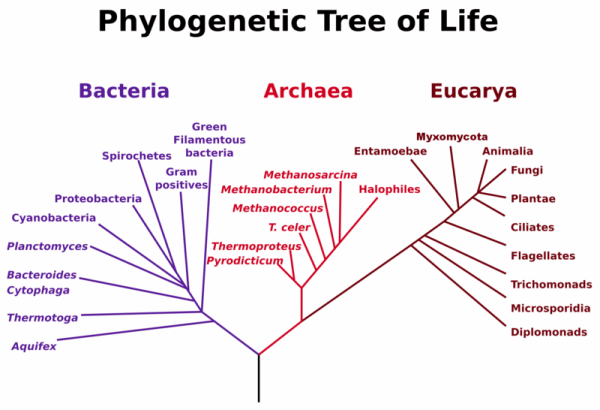
This file is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license
Archea often have very unique chemistries. Members of this domain can use not only carbohydrates and fats as sources of energy, but they can also use inorganic species such as ammonium, hydrogen, and metal ions as well as organic molecules such as methane. Some (methanogens) actually make methane. Archea were once thought to be found only in extreme environments (hence they were also called extremophiles), but in actuality they inhabit many environmental niches, including the oceans and soil. Since many do live in extreme environments, you would expect them to have evolved to synthesize stable, structural molecules. Archea use phospholipids in the membrane bilayers, but the lipids differ in three very important ways. Instead of fatty acid chains, they use isoprenoid chains as the nonpolar chains. Instead of using an ester link, the isoprenoids are covalently attached to the glycerol backbone with an ether link, which is obviously more stable than an ester bond used in the phospholipids discussed above. Finally, the stereochemistry of the phospholipids is based on sn-glycerol-1-phosphate and not sn-glycerol-3-phosphate.
Links and References
-
 Lipid
+ Genomics = Lipomics
Lipid
+ Genomics = Lipomics -
 Nature
Lipidomics Gateway
Nature
Lipidomics Gateway -
 Lipid
Metabolites and Pathways Strategies (LIPID MAPS)
Lipid
Metabolites and Pathways Strategies (LIPID MAPS) -
 Lipids:
General
Lipids:
General
Some References
- Mescar and Koshland. A new model for protein stereospecificity (other than 3 point binding). Nature. 403, pg 614 (2000)