Determination of Mechanism in Chemistry
Detection of Intermediates
DI2. Direct Observation
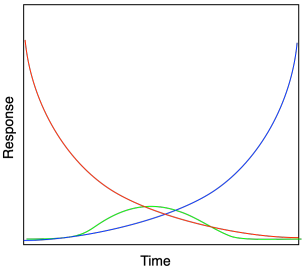
A mechanism is a sequence of intermediates that occur along a pathway from reactants to products. In some cases, an intermediate can be observed directly through experimental methods, and so a view of the mechanism can be built up around that piece of evidence. When following a reaction spectroscopically, we might be able to see the disappearance of a peak attributable to the reactant and the growth of a peak associated with the product. In a few fortunate cases, we might also see the temorary appearance of another set of peaks that then disappears, possibly indicating an intermediate in the reaction mechanism. If we can graph the absorbance or response of those peaks over time, we might see the decay of the reactant (red in the graph below), temporary appearance of the intermediate (green), and eventual appearance of the product (blue).

The detection of intermediates can be tricky, however. They typically don't last very long, so in most cases they don't build up to concentrations high enough to be detected easily. Compared to the intermediate, the reactant and product concentrations are much higher, obscuring any signal that may arise from transient structures along the mechanistic pathway. Observation of an intermediate often depends on the presence of a "spectral window": a region of the spectrum that isn't otherwise cluttered by other peaks, in which a peak due to the intermediate might be expected.
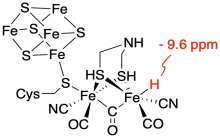
An example of such a case was demonstrated relatively recently by researchers at the Max Planck Institute for Chemical Energy Conversion and the Max Planck Institute for Coal Research, in Mulheim an der Ruhr, Germany.1 They were interested in looking for a proposed intermediate in an enzymatic reaction, the production of hydrogen gas by [FeFe] hydrogenase. A protein is a very large and complicated structure, so studying it spectroscopically is very challenging. Just being able to detect the relatively small active site would be daunting, let alone an intermediate within that active site. Nevertheless, these workers were able to use 1H NMR spectroscopy to observe a terminal iron hydride intermediate, Fe-H, that had been suggested as a possible reactive intermediate. It helped greatly that metal hydrides show up in a unique region of the NMR spectrum, near -10 ppm in this case.
Various spectroscopic methods a can be used to gain evidence of an intermediate during the course of a reaction. These approaches are usually limited by the very short lifetime of the intermediate. In some cases, methods can be used to prolong the lifetime of the intermediate. These methods include "matrix isolation" techniques, in which the intermediate is stabilized in an unreactive environment, often at low temperature. Typically the surrounding matrix must be transparent to spectroscopic methods, so that the intermediate can be observed without interference from the surrounding medium.
A number of valuable tools in science have been developed in pursuit of esoteric questions, goals that don't seem to have an immediately obvious application. In the 1960's and 1970's, one of those goals led scientists on a quest for the structure of cyclobutadiene. On paper, cyclobutadiene looks like a resonance-stabilized structure, much like benzene, but with a little more angle strain. Molecular orbital calculations suggested some deeper problems: the structure would be antiaromatic, with diradical character. The trouble was, nobody had managed to make a sample of cyclobutadiene (absent some experiments with chemical trapping), so its structure had never been examined. Cyclobutadiene was simply too reactive, and didn't last long enough to be observed.
Researchers at the University of Alberta decided to take a matrix isolation approach to the problem.2 They took a strained bicyclic lactone, which they hoped to use as a precursor to cyclobutadiene, and froze it in an argon matrix. Argon is a noble gas, of course; it is unreactive and therefore unlikely to interfere with any of the compounds under study. The researchers used a mercury lamp to irradiate the sample with UV light, to which the frozen argon glass is transparent. UV light triggers a retro-[2+2] cycloaddition (or a cycloreversion), freeing the cyclobutadiene.
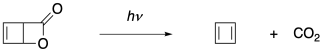
As a noble gas, argon is also non-molecular. It has no bonds. That fact allowed the team to study the products via IR spectroscopy, which is used to probe bonds in a sample and in this case would detect only those in the precursor, the carbon dioxide or the cyclobutadiene. They were chiefly interested in one question: is cyclobutadiene a square or is it a rectangle? If it behaved like an aromatic molecule, it would be a square, with equal bonds all the way around. If it behaved as though it contained two independent double bonds, somehow unable to conjugate with each other, it would be a rectangle. Those two shapes have different symmetries, and so group theory dictates a different number of IR peaks would be observed depending on the shape.
The result? IR spectroscopy revealed a rectangular cyclobutadiene. By being able to trap this highly elusive molecule in frozen argon, they were able to observe it directly and show that it does not behave like a π-delocalized molecule at all.
Problem DI2.1.
In a classic study, a Berkeley team used matrix isolation techniques to detect a coordinatively unsaturated transition metal intermediate capable of oxidative addition of C-H bonds.3
Cp*Rh(CO)2 was cooled to 242 K in liquid Kr; UV irradiation resulted in the disappearance of two IR bands near 2000 cm-1 and the appearance of a single band at 1947 cm-1. With the subsequent addition of cyclohexane, this peak shifted to 2003 cm-1.
a) What structural feature corresponds to the IR peaks followed in this study?
b) Why did the peak shift to lower frequency upon photolysis in liquid Kr?
c) Why did the peak shift to high frequency upon addition of alkane?
References:
1. Rumpel, S.; Sommer, C.; Reijerse, E.; Fares, C.; Lubits, W. Direct Detection of the Terminal Hydride Intermediate in [FeFe] Hydrogenase by NMR Spectroscopy. J. Am. Chem. Soc. 2018, 140, 3863-3866.
2. Masamune, S.; Souto-Bachiller, F. A.; Machiguchi, T.; Bertie, J. E. Cyclobutadiene Is Not Square. J. Am. Chem. Soc. 1978, 100, 4889-4891.
3. Weiller, B. H.; Wasserman, E. P.; Bergman, R. G.; Moore, C. B.; Pimental, G. C. Time-resolved IR spectroscopy in liquid rare gases: direct rate measurement of an intermolecular alkane carbon-hydrogen oxidative addition reaction. J. Am. Chem. Soc. 1989, 111, 8288-8290.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Determination of Mechanism
Back to Structure & Reactivity