PM12. Ion Exchange Chromatography
In ion exchange chromatography, we can separate ions from each other, largely based on their relative charges. If one ion is more highly charged than another, it will have more affinity for the stationary phase in an ion exchange column. It will spend more time on the stationary phase and move more slowly. A compound with a lower charge will elute first.
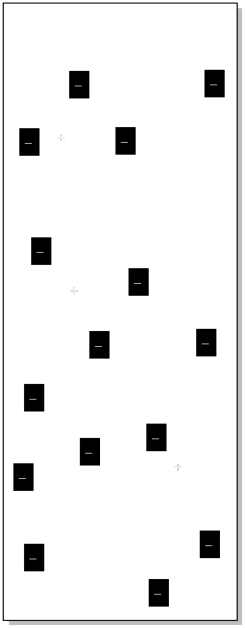
The stationary phase would have charges opposite those of the ions that we wished to separate. For example, maybe the column is packed with a phase that contains many anions. Maybe we have some sulfate ions attached to the packing in the column, each with a negative charge.

Figure PM12.1. The packing in an ion exchange column.
Suppose we have some cationic coordination complexes that we wish to separate from each other. We can dissolve them in water and introduce them to the column. Some of them have a charge of +1 and some of them have a charge of +2. If we introduced them to a stationary phase that contained a lot of anions, they both would stick to the stationary phase. The cations with the +2 charge would stick more strongly, however, and take longer to elute from the column. They would spend more time sitting still in the stationary phase and less time moving along in the mobile phase.
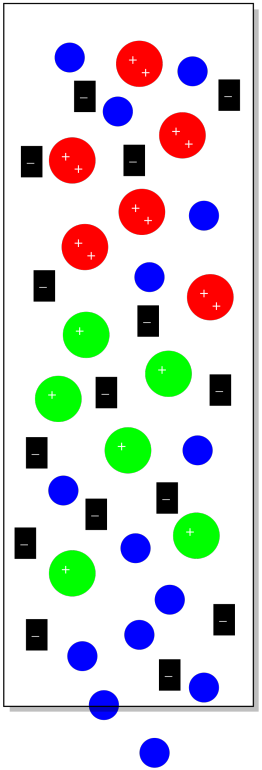
Figure PM12.2. Ion exchange chromatography.
Of course, there are some real problems with the picture so far. First of all, we can never have just one ion without a counterion. So those sulfate ions in the original stationary phase would have sodium ions, for example, and the sodium ions would balance out the charge.
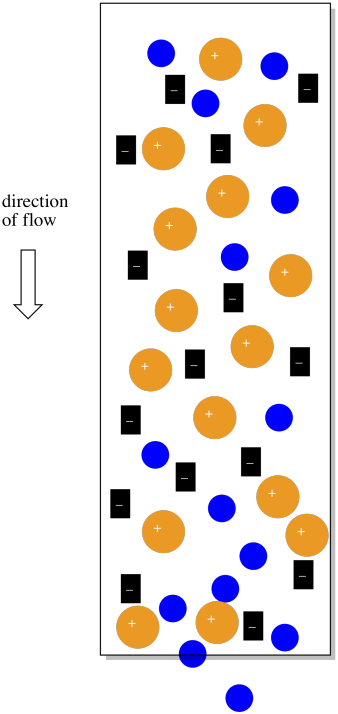
Figure PM12.3. The counterions in an ion exchange column.
The sodium ions will be very helpful in another way. If there are no counterions, why would the cationic complexes ever come off the column? They would just stick there forever. However, if the mobile phase is a salt solution, containing more sodium ions, then eventually the sodium ions will displace the coordination complexes, which will move along in the mobile phase for awhile.
Practically speaking, the eluted fractions will not contain pure coordination complexes. They will contain the mobile phase they came with when they eluted off the column, and that mobile phase contains some sodium salts. However, each coordination complex will be separate from the other one.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Structure & Reactivity