Silica and alumina are not the only possible solid phases. Stationary phases can be purchased that have long carbon chains bonded to silica beads. For example, a C18 column contains beads that have 18-carbon chains attached to them. These stationary phases are powders, like silica, and they can be loaded into a column just like silica can.
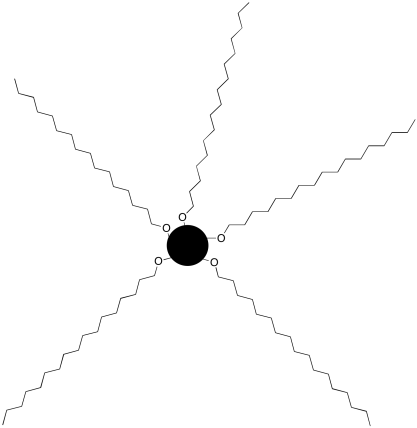
Figure PM10.1. A cartoon of a C18 bead.
A C18 column is an example of a "reverse phase" column. Reverse phase columns are often used with more polar solvents such as water, methanol or acetonitrile. The stationary phase is a nonpolar hydrocarbon, whereas the mobile phase is a polar liquid.
The same approach can also be used in TLC. If a plate is sprayed with a layer of C18 beads, then we can elute the plate in a polar solvent and separate compounds in a sample. Of course, things are reversed now. The most polar cpompounds will spend the most time in the mobile phase, and move most quickly. The least polar compounds will spend the most time in the stationary phase, and move most slowly.
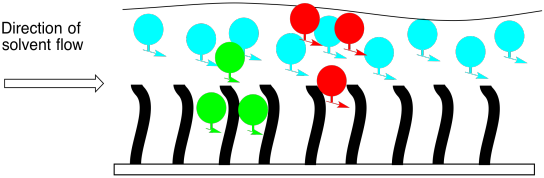
Figure PM10.2. A cartoon of reverse phase chromatography.
Problem 10.1.
On a silica (SiO2) column, three compounds were eluted in the following order using a hexanes / ethyl acetate mixture: p-dimethylbenzene, p-dimethoxybenzene, then p-methoxyphenol. What might you expect the order of elution would be on a C18 column?
Problem 10.2.
You are trying to elute a sample on a C18 column using 20:80 mixture of water:acetontrile, but the compounds are taking too long to come through the column. What should you do?
One of the advantages of reverse phase chromatography is that there are many kinds of stationary phase from which to choose. By changing the kind of chain that is to the bead, we can alter how strongly it will interact with certain molecules. Maybe a completely saturated hydrocarbon packing interacts well with saturated hydrocarbons in the sample, making them elute more slowly. On the other hand, maybe a more rigid packing that contains aromatics will interact better with aromatic hydrocarbons in the sample, making those elute more slowly. We might even have some polar groups mixed in, to get a mixture of interactions.
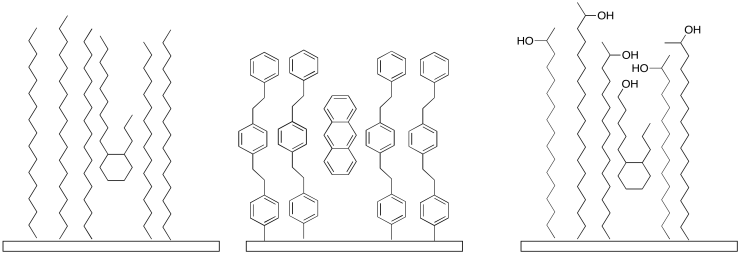
Figure PM10.3. There are many kinds of reverse phase packings.
Problem PM10.3.
Predict the order in which the following compounds would elute from a reverse phase column.
a) butylbenzene and benzylamine
b) 2-decanol and decanoic acid
c) 1-heptanol and 1-heptene
d) octanal and methyl octyl ether
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Structure & Reactivity