PM15. Solutions for Selected Problems
Problem PM1.1.
a) C6H5CO2H
b) HOCH2CH2OH
c) H2CO
Problem PM1.2.
a) Fastest: dichloromethane > hexane > water: slowest
a) Fastest: acetone > toluene > dimethyl sulfoxide: slowest
a) Fastest: pentane > ethyl acetate > benzene: slowest
Problem PM2.1.
a) Filter: sodium carbonate; filtrate: heptanal
a) Filter: benzophenone; filtrate: lithium chloride
a) Filter: anthracene; filtrate: potassium benzoate
a) Filter: tris(ethylenediamino)cobalt(III) chloride; filtrate: ethylenediamine
Problem PM3.1.
a) Yes, these compounds are both liquids and the boiling point difference is large.
b) No, these compounds are both liquids but the boiling point difference is small.
c) Yes, the compound you want to purify is a liquid and the boiling point difference is large. However, you would have to be very careful not to char the remaining material in the flask.
d) Yes, these compounds are both liquids and the boiling point difference is large.
e) No, these compounds are both solids. Distillation is not a good idea.
f) Yes, these compounds are both liquids and the boiling point difference is large. However, you would have to be careful not to char the desired material; as it distills (after the contaminant is removed), its volume will get smaller. At some point, heat dissipation will become a problem for the remaining material.
Problem PM5.1.
It’s possible that a mixture of sodium hydroxide and sodium oleate could be purified through the addition of water. If the right amount of water were added and the resulting slurry were stirred together and filtered, much of the sodium hydroxide would be removed because it is more soluble in water than is sodium oleate. Sodium oleate is less soluble in water than is sodium hydroxide, so most of it would not dissolve. It could be gathered or “isolated” by filtration.
Problem PM5.2.
Sodium oleate has a long, non-polar hydrocarbon chain. Hydrocarbon chains are not very soluble in water. That's beacuse they get in between the water molecules and prevent the water molecules from hydrogen bonding to each other.
Problem PM5.3.
The solution contains water, lots of sodium hydroxide, and a little sodium oleate.
Problem PM5.4.
No. If the water were evaporated, the sodium hydroxide would still be stuck in the sodium oleate.
Problem PM5.6.
You might have to filter out this impurity while everything else is still dissolved. You might have to make sure everythings stays warm while you do this. If things get too cold, the solubility will get lower, and compounds might solidify before you want them too. Sometimes, when filtering an aqueous solution, it helps to put some hot water in the filter flask and keep everything warm using steam.
Problem PM5.7.
The borneol seems too soluble in the methanol. If you don't have anymore borneol, you will have to evaporate the methanol again. This time, add less methanol; maybe you only need a quarter mL or ten drops or something. For a recrystallization to work, you want to see partial solubility; you want the compound to dissolve when hot but not when cold.
Problem PM5.8.
This is a good sign. Try heating the methanol to see whether more dissolves. If it dissolves when hot, try cooling it down and see whether the solid appears again.
Problem PM5.9.
This is still a good sign, but you might need to add more methanol to get it all dissolved. Don't forget to shake or stir it, too; that will help to get it dissolved.
Problem PM6.1.
Both water and ether (a common nickname for diethyl ether) contain electronegative oxygen atoms. Because both oxygens are bonded to less electronegative hydrogen or carbon atoms, each molecule will have a dipole. We may expect dipole-dipole interactions and miscibility.
Both oxygen atoms have lone pairs. Either one could act as a hydrogen bond acceptor. Because ether also contains a very polar O-H bond (remember, hydrogen bonding involves F,O,N), we may expect hydrogen bonding interactions and miscibility.
However, a C-O bond in ether is less polar than a H-O bond in water. In order for the two liquids to be miscible, stronger dipole-dipole interactions between the water molecules would have to be given up and traded in for weaker dipole-dipole interactions between the water and the ether molecules. The two liquids might not mix.
Ether has an oxygen atom with a lone pair, but it does not have a very polar O-H bond. The possibility for hydrogen bonding between water and ether is lower than between water molecules alone. The two liquids might not mix.
Ether has two hydrophobic hydrocarbon chains, although they are rather short. When mixing, these chains must be accommodated between neighbouring water molecules, which are thereby prevented from hydrogen bonding with each other. The two liquids might not mix.
Problem PM6.2.
The lighter ether would be on the top. The heavier water would sink to the bottom.
Problem PM6.3.
a) Given two separate layers, the lighter methanol would be on the top. The heavier water would sink to the bottom.
b) Methanol and water are miscible. Both are fully capable of hydrogen bonding; they are each hydrogen-bond donors and acceptors. Although the methanol has a non-polar hydrocarbon component, the methyl groups are not large enough to significantly disrupt hydrogen bonding between neighbouring water molecules.
Problem PM6.4.
a) Maybe the compounds are salts, containing an anion and a cation. The anions and cations could be simple inorganic ones such as Li+ and F-, but either the anion or the cation could also be organic (containing hydrocarbon portions). If the compounds are organic and not ionic, either the molecules would be fully capable of hydrogen bonding (containing O-H or N-H bonds), or they would contain highly polar bonds such as C=O. Also, the compounds should not contain too great a proportion of hydrocarbon compared to the polar part; for neutral compounds, that means a carbon : oxygen ratio below about 4 : 1, although the ratio can be significantly higher for ionic compounds.
b) The compounds should be neutral, not ionic. Although they may contain polar bonds, the compound should be mostly non-polar; a rough rule is that the carbon : oxygen ratio should be greater than 4: 1.
Problem PM6.5.
a) bottom b) top c) top d) top e) bottom f) top g) bottom h) top
Problem PM6.6.
The ionic compounds can form strong ion-dipole interactions with the water molecules. That interaction enhances their solubility in water.
Problem PM6.7.
Water and ether should be added to the mixture and the mixture should be shaken until it dissolves. The layers should be separated. The water should be extracted with additional ether and the combined ether layers should be washed with brine. The ether layers should be dried with sodium sulfate, filtered, and evaporated under vacuum.
Problem PM6.8.
The water molecules would have to come into contact with the sodium benzoate in order to dissolve it. If some sodium benzoate is completely surrounded by benzoic acid, it would remain undissolved.
Problem PM6.9.
Acetic acid, CH3CO2H, contains a polar C=O bond, a hydrogen-bonding O-H group, and a carbon: oxygen ration of 1:1. All of these factors render it relatively polar.
Problem PM6.10.
a) The THF is in the water layer.
b) The THF is in the ether layer.
a) The brine makes the water layer even more polar. The THF is already on the edge of being water-soluble, because it has a carbon : oxygen ratio of 4:1. The added water polarity pushes it past the tipping point.
Problem PM6.11.
After one extraction, half the perfluorobutanoic acid would remain in the water. A second extraction would remove half the remainder, leaving only a quarter of the original amount still in the water. A third extraction would leave 12% of the original in the water; a fourth extraction would leave 6%; a fifth extraction would leave 3%; a sixth extraction would leave 1.5% a seventh extraction would leave less than 1%.
The idea here is that multiple extractions are usually necessary. However, it would be pretty unusual to choose solvent partitioning as a purification method if a compound is this soluble in water.
Problem PM6.12.
a) It looks like half the ether has evaporated.
b) The white floaties are probably the bezoic acid that used to be dissolved in the ether. You don't have enough ether to keep it dissolved anymore.
c) You should add more ether before this experiment gets any worse.
Problem PM7.1.

Problem PM7.2.
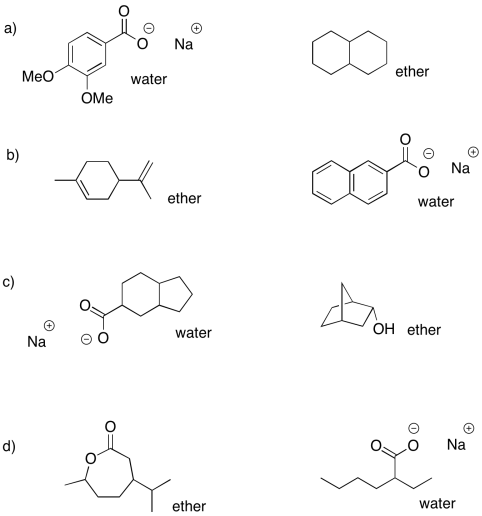
Problem PM7.3.
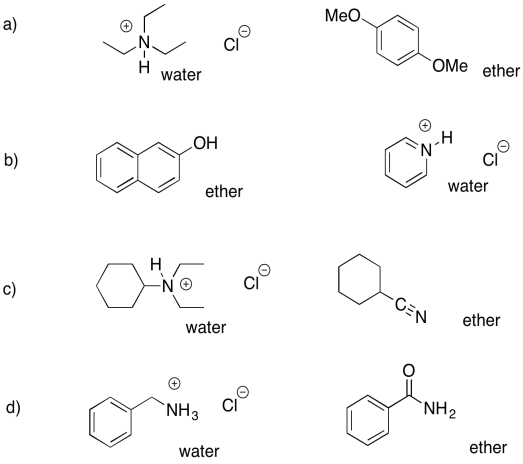
Problem PM7.4.
a) ether: cyclohexanone; water: sodium octanoate
b) ether: dibenzyl ether; water: benzylammonium chloride
c) ether: propyl heptanoate; water: sodium phenolate
d) ether: 2-octanol; water: sodium bromobenzoate
e) ether: heptanal; water: trioctylammonium chloride
f) ether: 4-methoxyphenol; water: sodium 3-nitrobenzoate
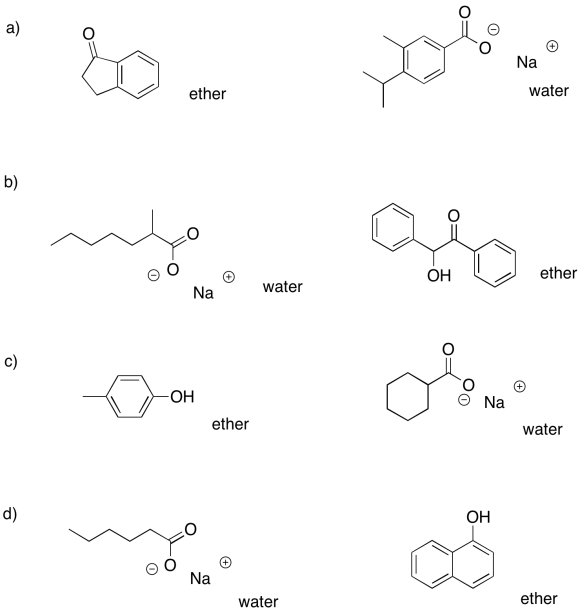
Problem PM7.5.
a) ethyl acetate (top): decanal; water (bottom): sodium benzoate
b) dichloromethane (bottom): benzyl alcohol; water (top): sodium 4-bromophenolate
c) ether (top): 3-heptanone; water (bottom): N,N-dimethylbenzylammonium chloride
d) chloroform (bottom): benzoic acid, 2-methylhexanoic acid; water (top): nothing
e) ether(top): nothing; water (bottom): sodium 2-ethylheptanoate, sodium 4-chlorophenolate
f) dichloromethane (bottom): benzonitrile, N,N-dimethyloctanamide; water (top): nothing
Problem PM8.1.
The cellulose contains many OH groups and can hydrogen bond with the water molecules.
Problem PM8.2.
Different pigments have different physical properties. Some of them will be more water- soluble than others. Some of them will adhere to the paper more strongly than others.
Problem PM8.3.
The pentane is not polar enough. You should add some 2-butanone to your solvent system.
Problem PM8.4.
The 2-butanone is too polar. You should add some pentane to your solvent system.
Problem PM8.5.
Sticking with the TLC method,you would probably want to start with a much larger plate than you used for your initial tests. Instead of putting a small dot of sample on the plate, you might paint a line of sample all the way across the plate. You would use exactly the same solvent system to elute the plate as one that you found worked well on a smaller scale. After eluting the plate, you would scrape off the three lines (not spots) that had separated on the plate. You would slurry each sample in some solvent that is pretty polar but evaporates easily (maybe the 2-butanone) and filter out the silica, then evaporate the solvent. You could put the remaining sample in a vial. repeat with the two other separated samples.
Problem PM9.1.
The method depends on adequate time for the compounds to differentiate from each other as they move through the column. If they all move too quickly, they are probably all spending too much time in the mobile phase.
Problem PM9.2.
Without flow to keep molecules moving forward, the compounds probably diffused in all directions while they were in the mobile phase. After some time, some compounds had probably spread throughout the column.
Problem PM9.3.
a) Instead of having a thin layer of sample at the beginning, we started with a very wide layer. As a result, by the time some molecules of one compound were emerging from the bottom of the column, others were just starting out at the beginning. Molecules of other compounds were already way ahead of them and they were not be able to catch up.
b) She kept all of the molecules together at the beginning so that molecules of one compound would all stay together and arrive at the end of the comlumn at the same time.
Problem PM9.4.
No. The solvent is more polar than the stationary phase in this case, so more polar compounds will spend more time in the mobile phase. As a result, more polar compounds will actually elute before less polar compounds.
Problem 10.1.
p-methoxyphenol, then p-dimethoxybenzene, then p-dimethylbenzene
Problem 10.2.
The compounds are not spending much time in the mobile phase, and have a much higher affinity for the non-polar stationary phase; use more acetonitrile and less water in the mobile phase.
Problem 10.3.
a) benzylamine then butylbenzene
b) decanoic acid then 2-decanol
c) 1-heptanol then 1-heptene
d) octanal then methyl octy ether
Problem 11.1.
a) brazzein then aspartame
b) rubsico then trypsin
c) titin then insulin
d) the MW 100,000 polystyrene then the MW 10,000 polystyrene
Problem PM15.1.
a) solvent and 1-hexanol
b) solvent and 1-propanol
c) solvent, methanol and 1-heptanol
Problem PM15.2.
a) hexene: [3,724 / (3,724 + 1,928)] x 100% = 65.9%;
2-bromohexane: [1,928 / (3,724 + 1,928)] x 100% = 34.1%
b) cyclooctene: [1,194 / (1,194 + 1,928)] x 100% = 38.2%;
1,2-cyclooctanediol: [1,928 / (1,194 + 1,928)] x 100% = 61.8%
c) pentene: [1,932 / (1,932 + 7,469 + 19,995)] x 100% = 6.6%;
2-pentanol: [7,469 / (1,932 + 7,469 + 19,995)] x 100% = 25.4%;
1-pentanol: [19,995 / (1,932 + 7,469 + 19,995)] x 100% = 68.0%
d) toluene: 100%
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Structure & Reactivity