SC2. Cis and Trans Stereoisomers
Two compounds that have the same formula and the same connectivity do not always have the same shape. There are two reasons why this may happen. In one case, the molecule may be flexible, so that it can twist into different shapes via rotation around individual sigma bonds. This phenomenon is called conformation, and it is covered in a different chapter. The second case occurs when two molecules appear to be connected the same way on paper, but are connected in two different ways in three dimensional space. These two, different molecules are called stereoisomers.
- There may be more than one way to arrange the same groups around the same atom with the same geometry.
- This phenomenon is called stereochemistry.

Figure SC2.1. Two stereoisomers. The atoms are connected to each other in the same order, but differ in their three-dimensional relationships.
One simple example of stereoisomers from inorganic chemistry is diammine platinum dichloride, (NH3)2PtCl2. This important compound is sometimes called "platin" for short. As the formula implies, it contains a platinum ion that is coordinated to two ammonia ligands and two chloride ligands (remember, a ligand in inorganic chemistry is an electron donor that is attached to a metal atom, donating a pair of electrons to form a bond).
Platin is an example of a coordination compound. The way the different pieces of coordination compounds bond together is discussed in the chapter of Lewis acids and bases.
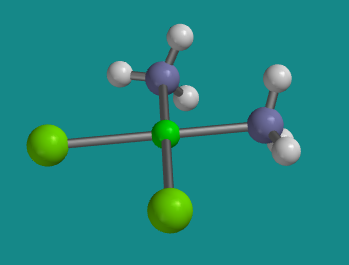
Figure SC2.2. Ball-and-stick model of cis-platin. This compound is square planar at platinum. It is flat when viewed from the edge, and square when viewed from the face.
Go to Animation SC2.1. A three-dimensional model of cis-platin.
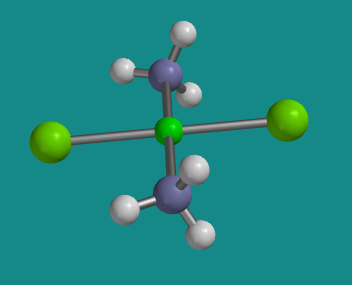
Figure SC2.3. Ball-and-stick model oftrans-platin. All the pieces are connected in the same way as in cis-platin, and the molecule is still square planar, but there is a different 3-dimensional arrangement.
Go to Animation SC2.2. A three-dimensional model of trans-platin.
For reasons arising from molecular orbital interactions, platin has a square planar geometry at the platinum atom. That arrangement results in two possible ways the ligands could be connected. The two sets of like ligands could be connected on the same side of the square or on opposite corners.
These two arrangements result in two different compounds; they are isomers that differ only in three-dimensional space.
-
The one with the two ammines beside each other is called cis-platin.
-
These two ligands are 90 degrees fom each other.
-
The one with the ammines across from each other is trans-platin.
-
These two ligands are 180 degrees from each other.
Although these two compounds are very similar, they have slightly different physical properties. Both are yellow compounds that decompose when heated to 270 degrees C, but trans-platin forms pale yellow crystals and is more soluble than cis-platin in water.
-
Cis and trans isomers have different physical properties.
Cis-platin has clinical importance in the treatment of ovarian and testicular cancers. The biological mechanism of the drug's action was long suspected to involve binding of the platinum by DNA. Further details were worked out by MIT chemist Steve Lippard and graduate student Amy Rosenzweig in the 1990's. Inside the cell nucleus, the two ammines in cis-platin can be replaced by nitrogen donors from a DNA strand. To donate to the Lewis acidic platinum, the DNA molecule must bend slightly. Normally that bend is detected and repaired by proteins in the cell. However, ovarian and testicular cells happen to contain a protein that is just the right shape to fit around this slightly bent DNA strand. The DNA strand becomes lodged in the protein and can't be displaced, and so it is unable to bind with other proteins used in DNA replication. The cell becomes unable to replicate, and so cancerous growth is stopped.
-
Cis and trans isomers have different biological properties.
Problem SC2.1. Draw the cis and trans isomers of the following compounds:
a) (NH3)2IrCl(CO) b) (H3P)2PtHBr c) (AsH3)2PtH(CO)
Problem SC2.2. Only one isomer of (tmeda)PtCl2 is possible [tmeda = (CH3)2NCH2CH2N(CH3)2; both nitrogens connect to the platinum]. Draw this isomer and explain why the other isomer is not possible.
