NMR5. Factors in Chemical Shift: Electronegativity
Electronegativity is a second factor that influences NMR spectra. The frequency of radio waves absorbed by an atom depends on the magnetic field experienced at the nucleus. The magnetic field experienced at the nucleus depends on the amount of electron density around the atom. Consequently:
- the more electron density present, the further upfield the shift in the spectrum.
- the less electron density present around the atom, the further downfield the shift.
Tributylamine has an NMR spectrum with four peaks, one for each inequivalent carbon in the structure. These peaks are spread out just a little bit more than in a hydrocarbon; there are more peaks showing up further downfield. The carbon next to the nitrogen is the one that shows up furthest downfield.
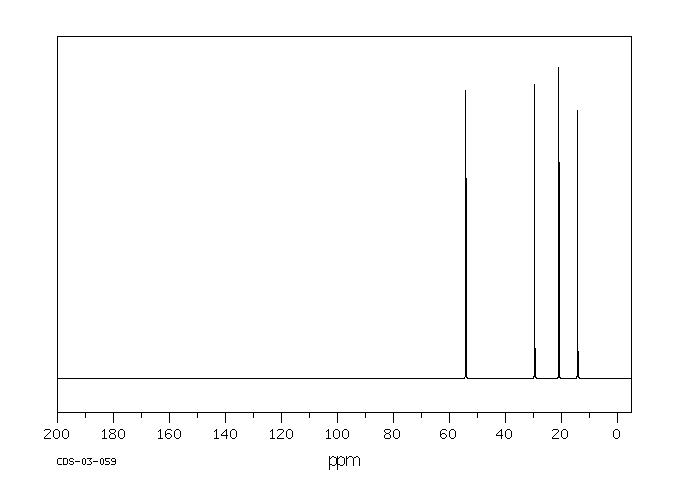
Figure NMR5.1. 13C NMR spectrum of tributylamine.
- usually, a tetrahedral carbon shows up in the upfield half of the spectrum.
- the region from about 0 to 100 ppm can be thought of as the sp3 window.
- an electronegative atom moves a peak further downfield within the sp3 window.
Dibutyl ether has peaks that show up even further downfield. As in tributylamine, the carbon next to the heteroatom, in this case an oxygen, shows up the furthest downfield. The other carbons in the chain also show up a little farther downfield than they would in butane, but the further from the oxygen they are, the less effect the oxygen has on them. This is a typical inductive effect. In an inductive effect, atoms have an effect on each other through sigma bonds, but the further apart the atoms are the smaller the effect.
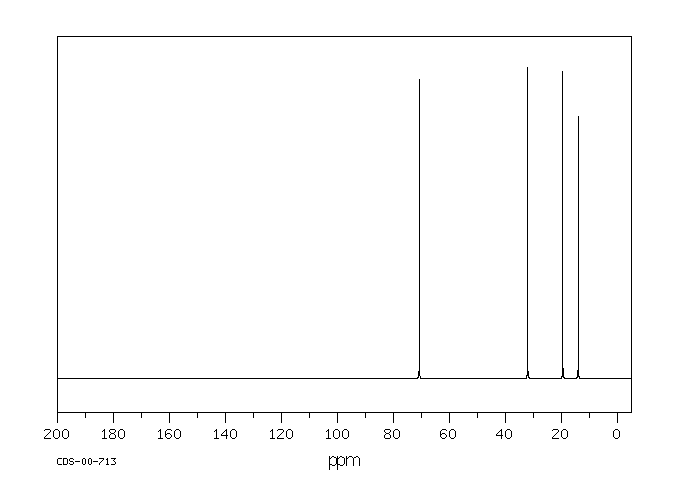
Figure NMR5.2. 13C NMR spectrum of butyl ether.
Notice that the absorbance of a carbon next to an oxygen atom was shifted even more to the left than a carbon next to a nitrogen atom. The more electronegative the neighbour, the greater the effect. You would probably expect a less electronegative neighbour than carbon will result in a shift to the right, and generally that's the case.
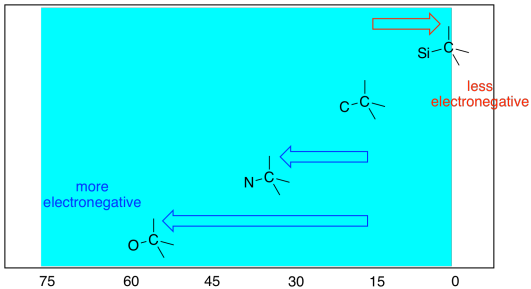
Figure NMR5.3. Shart showing electronegativity effects in the sp3 region of the 13C NMR spectrum.
In summary:
- electronegative elements draw attached carbons downfield.
- the more electronegative the element, the farther downfield the attached carbon.
- electronegative elements also have an effect on atoms further down the chain, drawing them downfield.
- the farther the atom is from the electronegative atom, the smaller the effect.
- the effect of electronegative atoms on their neighbours is called an inductive effect.
Methane (CH4) absorbs at about 5 ppm in the 13C NMR spectrum. Chloromethane absorbs at about 30 ppm. Since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar.
Dichloromethane or methylene chloride (CH2Cl2) shows up at about 55 ppm, and trichloromethane or chloroform (CHCl3) at about 80 ppm. The more bonds there are to an electronegative element, the further downfield the carbon absorbs. In the case of a chlorinated carbon, each additional chlorine moves the peak about 25 ppm further downfield.
- the effect of electronegativity is additive.
- the more electronegative elements attached to a carbon, the farther downfield it absorbs.
These trends are also seen among sp2 carbons. An oxygen atom attached to an sp2 carbon results in a downfield shift, to about 160 ppm. On the other hand, if a trigonal planar carbon is double bonded to an oxygen atom, the shift can be much farther; it actually ranges from 160 to 210 ppm, depending on what else is attached to the carbon, and is most commonly seen around 180 ppm.
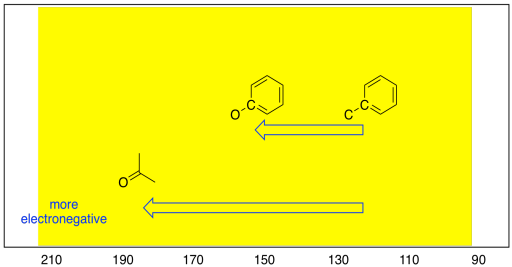
Figure NMR5.4. Shart showing electronegativity effects in the sp2 region of the 13C NMR spectrum.
Problem NMR5.1.
Suggest an assignment for the following 13C NMR peaks:
a) 12 ppm b) 58 ppm c) 22 ppm d) 41 ppm
Problem NMR5.2.
Draw the predicted 13C NMR spectra for the following compounds.