Reactivity in Chemistry
Ligand Substitution in Coordination Complexes
LS2. Mechanistic Possibilities
There are two basic steps in ligand substitution: association and dissociation. Association, in this case, refers to the binding of a ligand to the metal. The ligand donates an electron pair to the metal and the two molecules come together to form a new bond. Dissociation, in this case, refers to the release of a ligand from a metal. The metal-ligand bond breaks and the ligand leaves with its electron pair.
Two mechanistic possibilities seem pretty obvious. Either the new ligand binds first and then the old one leaves, or the old ligand leaves first and then the new one binds.
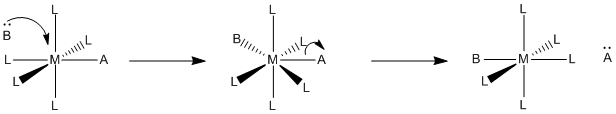
In an associative mechanism, the new ligand starts the reaction off by associating with or binding to the transition metal. That's the bond-making step. The bond-breaking part, or dissociation, comes later.

Figure LS2.1. An associative substitution mechanism.
In a dissociative mechanism, the old ligand goes ahead and dissociates from the transition metal before the new ligand has arrived. That's the bond-breaking step. The bond-making part, or association, comes later.
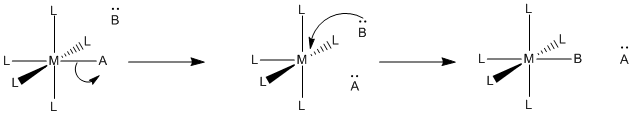
Figure LS2.2. A dissociative substitution mechanism.
Knowing the mechanism is important because the mechanism has an impact on what factors affect the reaction. For example, if the reaction is associative, adding lots more new ligand may speed up the reaction, because then it becomes more likely that the new ligand will find the metal complex and bind with it. However, if the old ligand is supposed to leave before the new ligand arrives, then it doesn�t matter how much new ligand is around. It has to wait for the old ligand to leave before it can bind, anyway, so adding a lot more new ligand won't speed things up.
Problem LS2.1.
a) Which kind of step costs more energy: bond-making or bond-breaking?
b) What would be the rate-determining step in the associative mechanism?
c) What would be the rate-determining step in the dissociative mechanism?
d) What would be the rate law for the associative mechanism?
e) What would be the rate law for the dissociative mechanism?
Problem LS2.2.
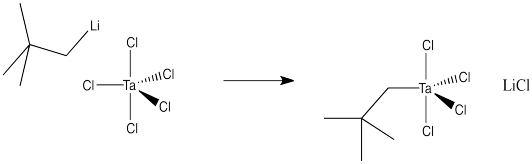
Draw a mechanism, with arrows, for the following substitution. Assume an associative mechanism.
Problem LS2.3.
Draw a mechanism, with arrows, for the following substitution. Assume a dissociative mechanism.

Problem LS2.4.
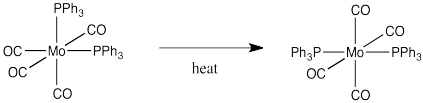
Draw a mechanism, with arrows, for the following isomerization.
Problem LS2.5.
The ability to substitute for a ligand depends partly on its ability to leave. Rank the following ligands, from the easiest to replace to the hardest to replace:
CO Cl- PPh NH3 NO3- H2O
Problem LS2.6.
Draw curved arrows for the following steps. Classify each step as associative or dissociative.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Ligand Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry