Reactivity in Chemistry
Electrophilic Aromatic Substitution
AR9. Aryl Diazonium Reactions
The introduction of a side group on a benzene ring is just one aspect of benzene reactivity. Often, these groups undergo unique reactions that can be synthetically useful.
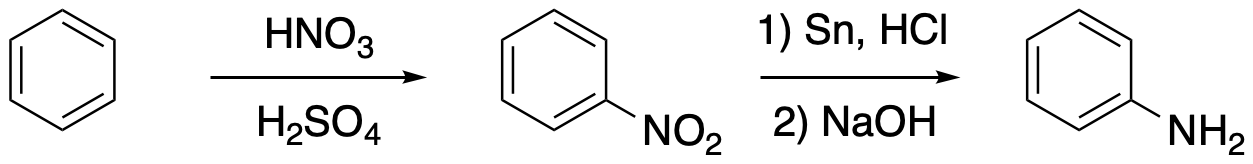
In this section, we will look at a series of reactions in which an arylamine group can be modified in different ways. The amine group comes from a nitro group, which is added via nitration of the aromatic in nitric and sulfuric acid. The nitro group is then converted to an amine via reduction with HCl and an active metal, such as tin or iron. Once it is in place, the amine group can be converted to an aryl diazonium group, Ar-NN+ X-. These diazonium ions undergo a range of reactions in which dinitrogen (N2) is displaced by an apparent nucleophile. They also undergo another kind of reaction in which the N2 group attaches to another benzene ring, forming a bridge between two benzenes. This is called a diazonium coupling reaction. We'll look at these coupling reactions later. Before that, we're going to look at the substitution reactions. But to start off, we're going to see how to make the diazonium salt that is used in both of those reaction types.

Figure AR9.1. Formation of nitrobenzene and conversion to phenylamine (aniline).
Converting an arylamine to a diazonium salt involves generation of a nitrosyl cation, NO+, as an electrophile in situ. That means we add a mixture that reacts together to make the electrophile and that electrophile then reacts with the amine nucleophile. Generating an electrophile in situ is something we did a number of times in electrophilic aromatic substitution reactions. This time, generating the electrophile is a lot like generating the nitronium cation, NO2+, in an aromatic nitration. We add sodium nitrite, NaNO2, in a strong acid such as sulfuric or hydrochloric acid. The nitrite ion gets protonated and loses water to form the electrophile. After that, the amine nucleophile acts do displace the oxygen from the nitronium ion in the form of water.
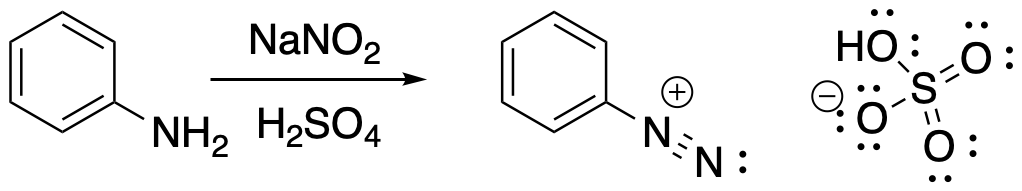
Figure AR9.2. Conversion of aniline to a diazonium salt.
Problem AR9.1.
Draw a mechanism with intermediates and curved arrows for the formation of the NO+ electrophile from NaNO2 and H2SO4.
Problem AR9.2.
Draw a mechanism with intermediates and curved arrows for the reaction of the NO+ electrophile with aniline (PhNH2).
The basic way to remember what happens with reactions of aryldiazonium ions is to think of that N2 group as a leaving group. Arydiazonium ions undergo substitution reactions in which an apparent nucleophile replaces a nitrogen leaving group. However, the reaction mechanism isn't necessarily that simple.
The first group of these reactions we will look at just replaces the dinitrogen group with a halogen atom. Maybe this doesn't seem like the most useful reaction, given that we already know how to puts halogen atoms on a benzene ring. We can treat benzene with bromine or chlorine and a Lewis acid to add a Br or a Cl, respectively. Now we see that we can also treat an aryl diazonium salt with copper(I) salts, copper(I) bromide or copper(I) chloride, to accomplish the same thing. We can do the same thing with copper(I) iodide to add an iodine atom to the benzene, which is something we couldn't do before. Adding iodine works even better if we just add NaI or KI, but some textbooks use the cuprous iodide to make it easier to remember, since we are already using cuprous salts for the other halogens. These copper-mediated substitutions are often called Sandmeyer reactions.
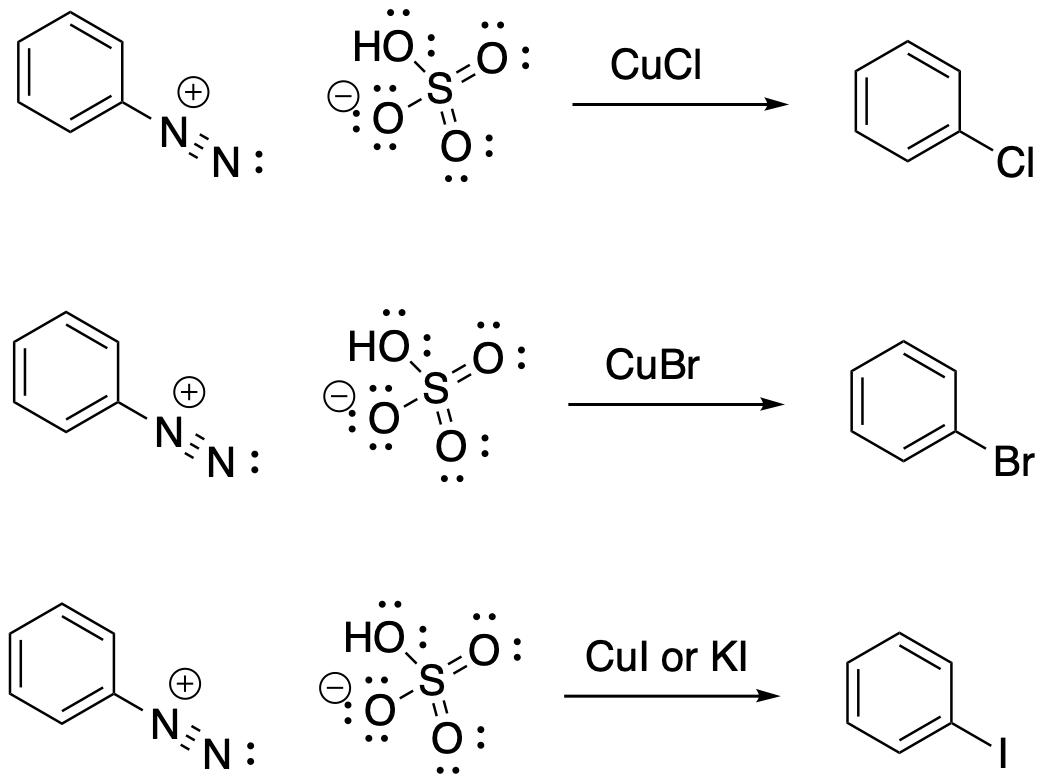
Figure AR9.3. Conversion of a diazonium salt to a chlorobenzene, bromobenzene, and iodobenzene.
There is also a Sandmeyer reaction that allows us to add a nitrile group to the benzene. Remember, nitriles were another example of meta-directing, deactivating groups in aromatic substitution. A nitrile is attached to the benzene by treating the diazonium salt with cuprous cyanide, CuCN.
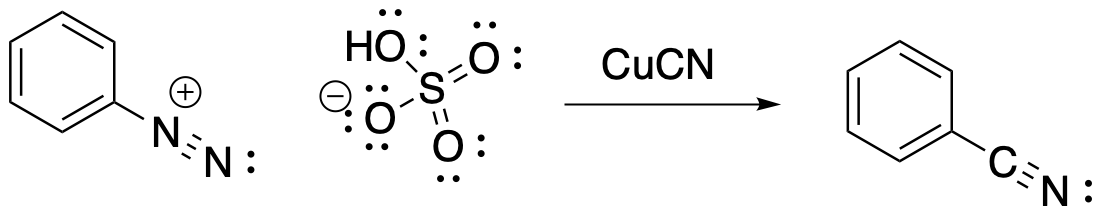
Figure AR9.4. Conversion of a diazonium salt to benzonitrile.
One more copper variation. If we want to attach an OH group to the benzene, we can do so by adding a gemisch that contains Cu2O, Cu(NO3)2, and H2O. That's a mixture of copper(I) and copper(II) salts.
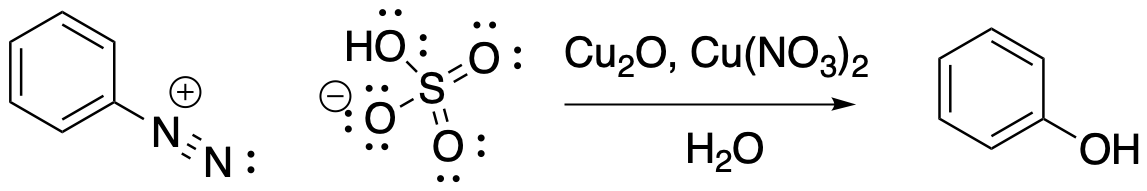
Figure AR9.5. Conversion of a diazonium salt to a phenol.
The mechanisms of these reaction are a little unclear, but they are generally believed to involve radical intermediates. Radicals are reactive species with single, unpaired electrons. Reactions involving single electrons, rather than the pairs of electrons that we're more familar with, often show up when we are dealing with copper. However, these reactions also seem to involve cationic intermediates; there seem to be a number of different reactive intermediates along the pathway. That makes the mechanism fairly complicated, so we're not going to go into it any further.
We can also use a diazonium reaction to add fluorine to a benzene ring. That's another variation we couldn't do using the same Lewis acid strategy that we use with chlorine and bromine. This time we don't use a copper salt, though. Instead, we treat the diazonium salt with sodium tetrafluoroborate, NaBF4. This variation is often called a Schiemann reaction.
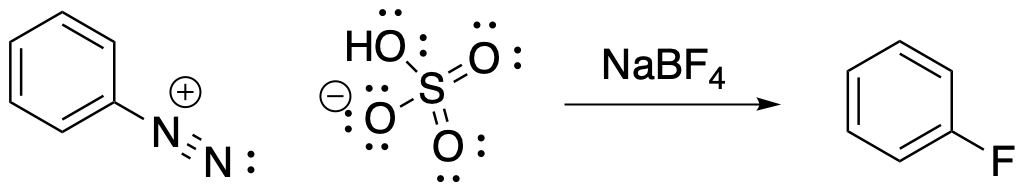
Figure AR9.6. Conversion of a diazonium salt to a fluorobenzene.
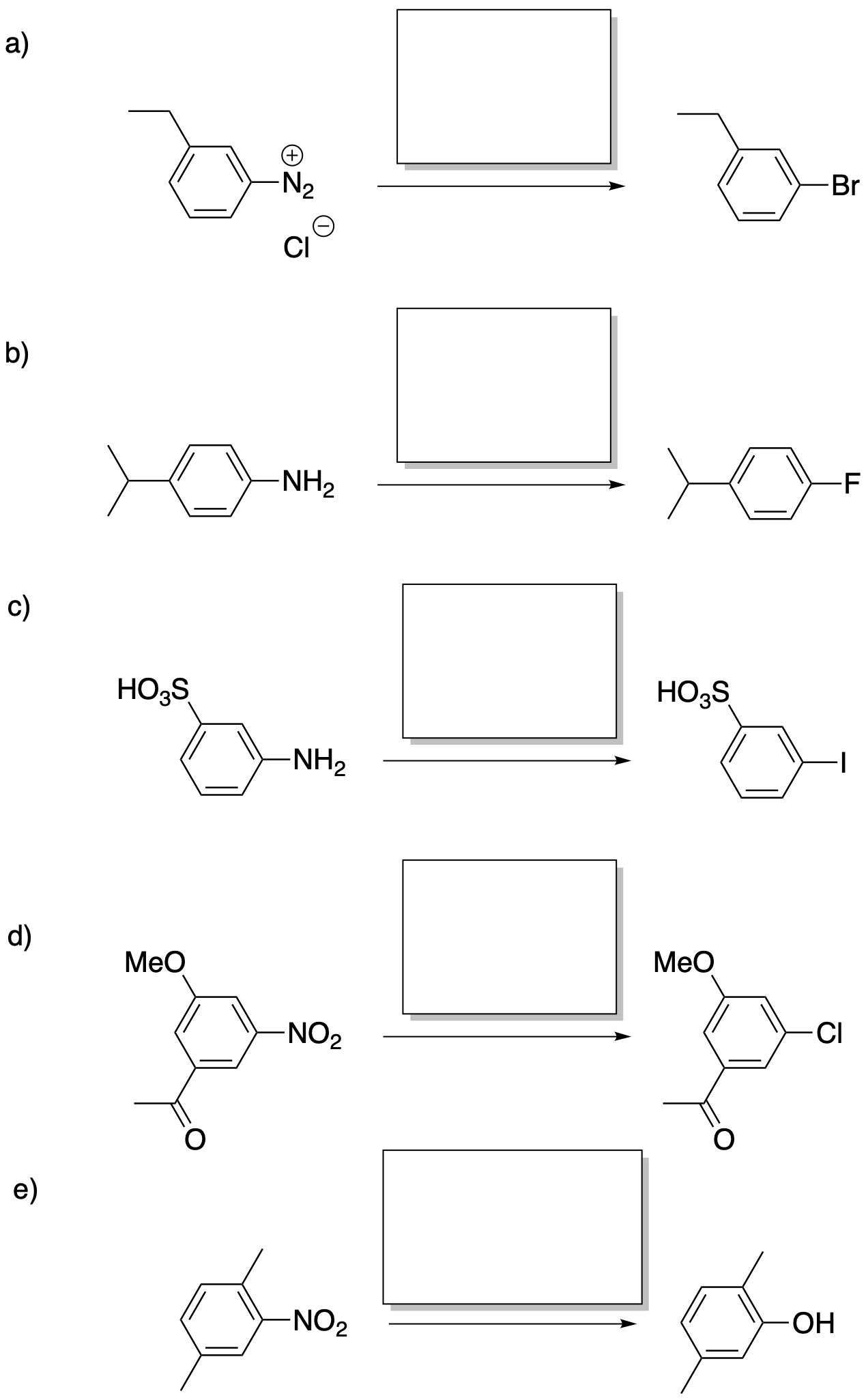
Problem AR9.3.
Fill in the missing reagents.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: