Reactivity in Chemistry
Electrophilic Aromatic Substitution
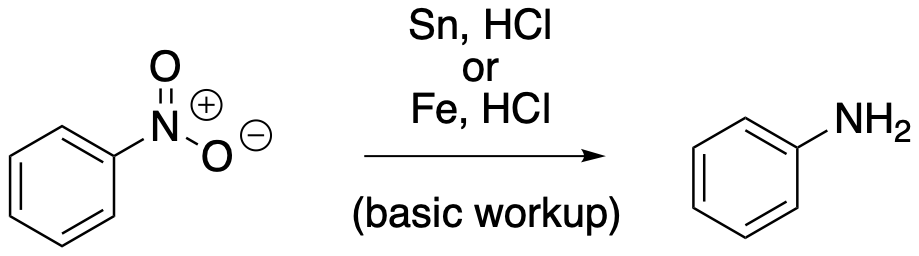
AR7. Side Chain Reduction: Nitro Groups
The introduction of a side group on a benzene ring is just one aspect of benzene reactivity. Often, these groups undergo unique reactions that can be synthetically useful. Oxidation and reduction reactions are frequently used to modify the side chains of aromatic compounds. In organic chemistry, oxidation reactions often involve the formation of new bonds between carbon and oxygen. Reduction reactions often involve the formation of new bonds between carbon and hydrogen.
In this section, we will look at some reduction reactions in which oxygen atoms at the position next to the aromatic ring are replaced by hydrogen atoms. The replacement of an oxygen atoms by a hydrogen atom is considered a reduction. In oxidation state formalisms, a hydrogen is considered to have a 1+ charge, whereas an oxygen is considered to have a 2- charge. To balance that charge, an atom attached to hydrogen would have a more negative oxidation state (or a less positive one) if attached to hydrogen but a more positive oxidation state (or a less negative one) if attached to oxygen.
If we are replacing oxygen with hydrogen, we need a source of hydrogen atoms. In the reactions we will see here, the hydrogen is supplied in the form of protons from an aqueous acid. Typically, that means HCl in these cases. If we are reducing the atom that was attached to oxygen, it means we will also need to supply electrons to put the atom in a lower oxidation state. In these reactions, we will supply electrons through the use of an active metal. An active metal is one that can give up electrons relatively easily. There are a number of possibilities, but we will see the use of tin (Sn), iron (Fe) and zinc (Zn) in these examples.
Nitro groups can be added to benzene rings by treatment with a mixture of nitric acid in sulfuric acid. Nitro groups are strong deactivators of electrophilic aromatic substitution reactions, so that once an aromatic ring has a nitro group attached to it, further electrophilic aromatic substitution slows down substantially, or even stops altogether. Amino groups, on the other hand, are completely different. They are strongly activating towards electrophilic aromatic substitution. Furthermore, nitro groups are meta-directing in electrophilic aromatic substitution reactions, whereas amino groups are ortho- and para-directing. We know amino groups are very different than nitro groups, but we haven't seen how an amine can be attached to a benzene ring.
A nitro group can be converted into an amino group pretty easily. The most common method of carrying out this conversion is with a moderately active metal and aqueous acid. An active metal is one that can give away electrons. Examples that work in this case include tin (Sn), zinc (Zn), iron (Fe), and indium (In). The acid most commonly used for this purpose is hydrochloric acid (HCl). It's worth noting that the nitro group does not have to be attached to a benzene for this reaction to work; it also works with aliphatic or alkylamines. However, it's much easier to attach a nitro group to a benzene than to an alkyl chain: we just throw the benzene in that nitric-sulfuric acid mixture. As a result, reduction of the nitro group to an amine is something you'll more often see in the case of aromatic compounds, leading to the formation of aromatic amines (commonly called anilines).

Figure AR7.1. Reduction of a nitrogen with active metals.
Remember, a reaction leading to the formation of an amine is often followed by a basic workup. This step is especially important when the reaction conditions involve the use of acid. Under acidic conditions, amines are easily protonated. They form ammonium salts. In this case, in the presence of hydrochloric acid, the arylamine would form an arylammonium hydrochloride salt. That's a problem for a couple of reasons. At the simplest level, if it's an amine that you want, you haven't got it: you've got a related compound, but it isn't the same thing. If it isn't the same thing, it has different physical and chemical properties, so it isn't going to behave the way you want it to. Most importantly in this case, the hydrochloride salt will be water-soluble because of strong ion-dipole interactions with the solvent. Usually, after an organic reaction, we add water to wash away any inorganic salts. This is called the reaction workup. If we just added HCl and Sn, we'll wash away any tin salts that formed, such as SnCl2, plus any leftover HCl. But if the aniline has formed a hydrochloride salt, we're going to wash that way as well. We need to make sure we turn that hydrochloride salt back into a regular amine so it doesn't get lost in the water layer.
The mechanism of this reaction has not been worked out in detail. Nevertheless, the simple reagents suggest a general idea of how it must work. At a very simple level, we need to replace the two oxygen atoms on the NO2 group with the two hydrogen atoms that would make it an NH2 group. Well, a hydrogen atom is just a proton and an electron. In this reaction, the HCl supplies the protons and the metal, such as Fe, supplies the electrons, leaving behind ferrous chloride salt, FeCl2.
Fe → Fe2+ + 2 e-
2 H+ + 2 e- → 2 H
Combining those things:
2 H+ + 2 Cl- + Fe → 2 H + FeCl2
In terms of a mechanism, we often think of similar reductions as occurring through alternating proton and electron transfers. Biochemists use the term "proton-coupled electron transfer" to describe situations in which the proton and electron are coming from two different places but their movement seems in some way coordinated with each other. This mechanism might involve something like that. We often think of the proton as moving first, simply because it is bigger and heavier and it moves more slowly because of greater inertia. The electron is slower and lighter and moves more quickly. As soon as a proton is added to the nitro compound, the nitro compound will gain a positive charge. That charge will attract the electron very quickly. We know that oxygen is not a very good leaving group, but at least in simple molecules that problem can be remedied under acidic conditions; if we can protonate the oxygen, we can turn it into a neutral water leaving group. Combining all of those ideas, we get a series of protonations and electron transfers to displace water, reduce the nitrogen (add electrons to it), and protonate the nitrogen to form an amine.
Problem AR7.1.
Problem AR7.2.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Navigation: