Reactivity in Chemistry
Electrophilic Aromatic Substitution
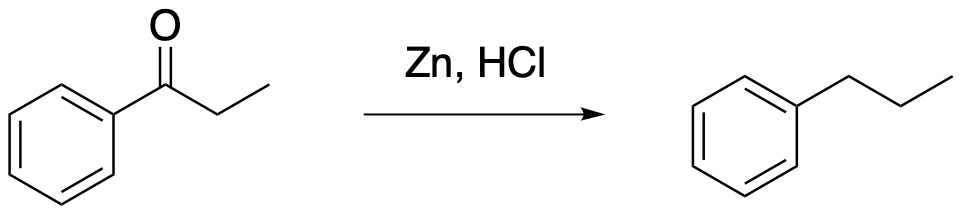
AR8. Side Chain Reduction: Ketones
The introduction of a side group on a benzene ring is just one aspect of benzene reactivity. Often, these groups undergo unique reactions that can be synthetically useful. Oxidation and reduction reactions are frequently used to modify the side chains of aromatic compounds. In organic chemistry, oxidation reactions often involve the formation of new bonds between carbon and oxygen. Reduction reactions often involve the formation of new bonds between carbon and hydrogen.
In this section, we will look at some reduction reactions in which oxygen atoms at a ketone position next to the aromatic ring are replaced by hydrogen atoms. The replacement of an oxygen atoms by a hydrogen atom is considered a reduction. In oxidation state formalisms, a hydrogen is considered to have a 1+ charge, whereas an oxygen is considered to have a 2- charge. To balance that charge, a carbon attached to hydrogen would have a more negative oxidation state (or a less positive one) if attached to hydrogen but a more positive oxidation state (or a less negative one) if attached to oxygen. For example, if the carbonyl carbon is attached to two other carbons (it's a ketone), then those two other carbons wouldn't affect it's oxidation state. Bonding to another carbon wouldn't change the amount of electron density on the carbonyl carbon. What really changes the electron density on that carbonyl carbon is the attached oxygen. If we went to the extreme of thinking of the C=O bond as ionic, we would think of the oxygen as O2- and the carbon as C2+.
However, if we had an alkyl group attached to the benzene then that first carbon, the benzylic carbon, would not be thought of as C2+. In fact, if we thought of the C-H bonds as ionic, we would think of the hydrogens as H+. If there were two C-H bonds, we would think of both hydrogens as H+ and the carbon as C2-. If we could transform a ketone into an alkyl group, we would turn a C=O into a CH2. We would turn the C2+ into a C2-. We would be adding negative charge to the carbon, which is considered a reduction.
If we are replacing oxygen with hydrogen, we need a source of hydrogen atoms. In the reactions we will see here, the hydrogen is supplied in the form of protons from an aqueous acid. Typically, that means HCl in these cases. If we are reducing the atom that was attached to oxygen, it means we will also need to supply electrons to put the atom in a lower oxidation state. In these reactions, we will supply electrons through the use of an active metal. An active metal is one that can give up electrons relatively easily. In the reduction of a C=O group to a CH2 group, we almost always use zinc (Zn) as the active metal. The zinc is usually mixed with mercury to form an amalgam (that's just a mixture of metals), often written as Zn(Hg). Other active metals that can reduce nitro groups, such as iron and zinc, don't work on ketones.
This method is commonly called a Clemmensen reduction. It works on other ketones, too. There doesn't need to be a benzene next to it, although that's the situation we see most often when people use this reaction.

Figure AR8.1. Reduction of a ketone with an active metal, zinc.
The mechanism of this reaction isn't completely understood. Nevertheless, the reagents suggest a general idea of how it must work. At a very simple level, we need to replace the oxygen atom on the C=O group with the two hydrogen atoms that would make it an CH2 group. Remember, a hydrogen atom is just a proton and an electron. In this reaction, the HCl supplies the protons and the Zn metal supplies the electrons, leaving behind zinc chloride salt, ZnCl2.
Zn → Zn2+ + 2 e-
2 H+ + 2 e- → 2 H
Combining those things:
2 H+ + 2 Cl- + Zn → 2 H + ZnCl2
We often think of similar reductions as occurring through alternating proton and electron transfers. We usually imagine the proton as moving first, simply because it is bigger and heavier and it moves more slowly because of greater inertia. The electron is slower and lighter and moves more quickly. As soon as a proton is added to the carbonyl compound, the carbonyl will gain a positive charge and become activated. That charge will attract the electron very quickly. We know that oxygen is not a very good leaving group, but at least in simple molecules that problem can be remedied under acidic conditions; if we can protonate the oxygen, we can turn it into a neutral water leaving group. Combining all of those ideas, we get a series of protonations and electron transfers to displace water, reduce the carbon (add electrons to it), and protonate the carbon to form a methylene or CH2 group.
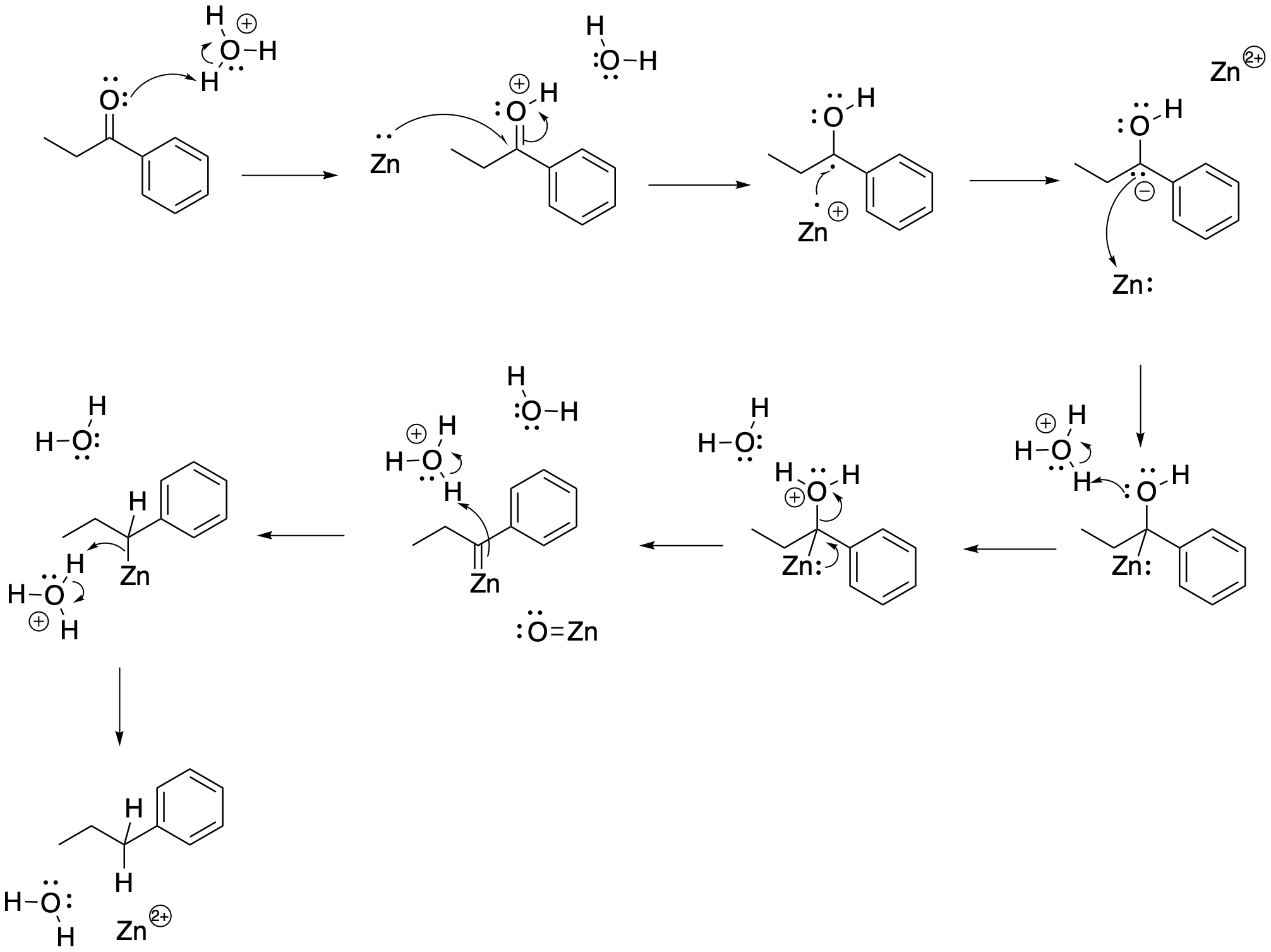
Figure AR8.2. Possible mechanism for reduction of a ketone with zinc.
There is just one unusual mechanistic detail in this case. Studies of the Clemmensen reaction suggest the involvement of a specific kind of intermediate called a metal carbene. The specific type of carbene seen here is sometimes called a Schrock carbene or alkylidene. This intermediate contains a metal carbon double bond, in this case C=Zn. This intermediate occurs rather than a highly reactive carbon anion. In a sense, the zinc is helping to stabilize this reactive carbon species.
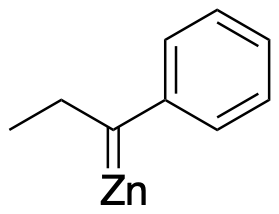
Figure AR8.3. A zinc alkylidene intermediate.
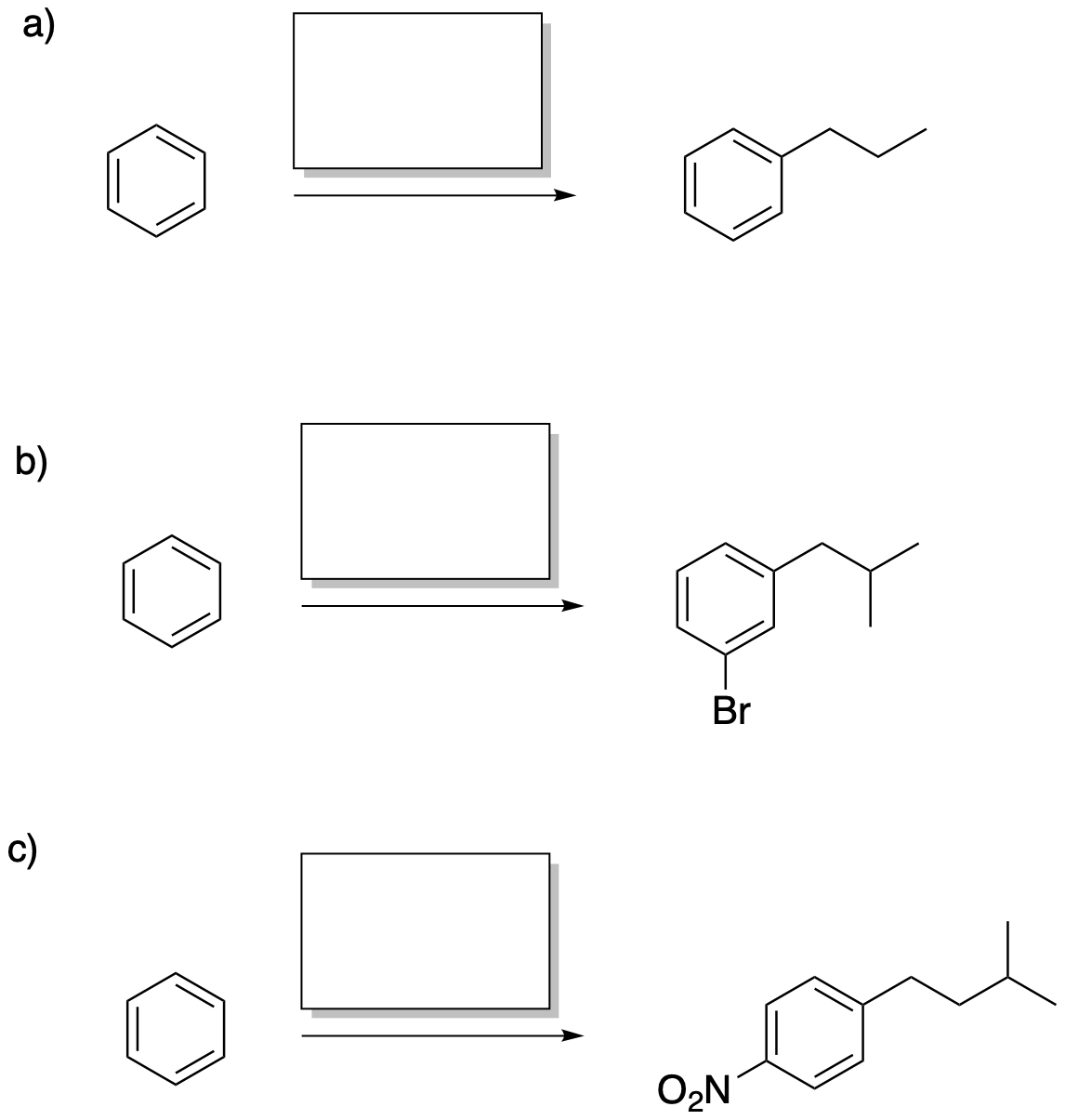
Problem AR8.1.
Problem AR8.2.
Provide the regents, or sequences of reagents, needed to carry out the following transformations.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Navigation: