Reactivity in Chemistry
Reduction Potentials of Metal Ions in Biology
MB3. Effect of Charge: Distal Effects and Effects of the Medium
The charge on a coordination complex, found within a metalloprotein, has a strong influence on the reduction potential of the metal centre. Other factors in the surrounding medium can either stabilise or destabilise that charge. Consequently, we need to consider the interaction of the metal centre with its surroundings.
Consider a metal centre in which the local charge -- that is, the sum of the charges on the metal ion and its coordinated ligands -- is -2. The addition of an electron would result in a local charge of -3.
But why are we talking about this "local charge"? Isn't -3 the charge on the whole molecule? Well, no. Remember, we are talking about a protein. It is a very large molecule. What's more, it has lots of other charges associated with it. Many amino acid residues may be neutral, but some have negative charges and others have positive charges.
Those charges are bound to interact with the charge on the metal centre. There would be coulombic interactions between the charges. Think about a case in which there are a number of nearby, positively charged amino acid residues.

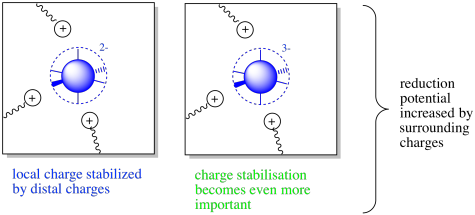
Figure MB3.1. The oxidized (left) and reduced state (right) of a coordination complex with surrounding charges.
Those positively charged residues would have an attractive interaction with the metal centre. The overall energy would be lowered by this interaction. If another electron were added to the metal centre, and it became more negative, the interaction with the neighbouring positive charges would be even greater. As a result, a balance is tipped within this protein. The positively charged residues stabilize both the oxidized and the reduced state of the metal centre, but they stabilize the reduced state even more. Reduction potential increases in this case.
Problem MB3.1.
Which amino acid residues are generally found with positive charges at neutral pH?
Now let's think about the opposite case. We'll take the same metal centre, but surround it with some negatively charged amino acid residues.
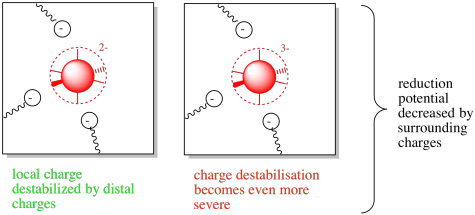
Figure MB3.2. The oxidized (left) and reduced state (right) of a coordination complex with surrounding charges.
In this case, the neighbouring amino acid residues would destabilise the charge on the metal centre. However, if the metal became reduced, the situation would become even worse. The buildup of negative charge would lead to increasing repulsive and destabilising forces. The reduction potential would be lowered.
Problem MB3.2.
What amino acid residues are generally found with negative charges at neutral pH?
Of course, not all protein-metal complexes have a negative charge around the metal ion. If the coordinating donors are neutral rather than anionic, the complex may have an overall positive charge. How will that change things?
Well, the details do change, but the overall idea remains the same. This time, surrounding amino acid residues will stabilize the complex only if they are negatively charged. Of course, the more positively charged the complex, the more important that interaction. This situation may result in a decreased reduction potential.
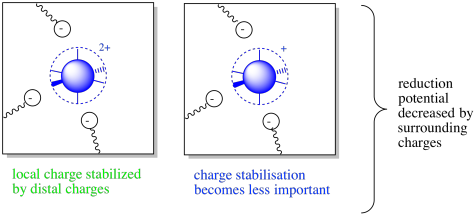
Figure MB3.3. The oxidized (left) and reduced state (right) of a coordination complex with surrounding charges.
That situation would be reversed in the presence of nearby amino acid residues with positive charges. In that case, the destabilization of the more positive oxidized state may motivate the complex toward accepting an electron. The reduction potential may be increased in this case.
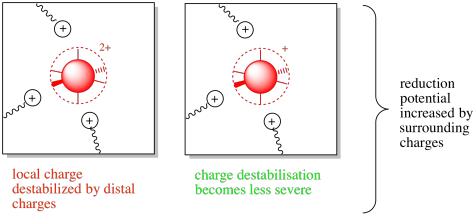
Figure MB3.4. The oxidized (left) and reduced state (right) of a coordination complex with surrounding charges.
All of these examples involve just a few amino acid residues that are actually charged at neutral pH or thereabouts. Does that mean that these factors are rare, only to be considered when there happen to be such residues close enough to actually make a difference?
Not really. Remember, a protein has lots of polar aspects. Even in the absence of charges, there are a number of amino acid residues that would have dipole moments. These dipole moments would have ion-dipole interactions with charged metal centres. In general, neighbouring dipoles would be able to stabilise charges, provided the amino acid residues were conformationally flexible enough to interact with the charge in a stabilising way.
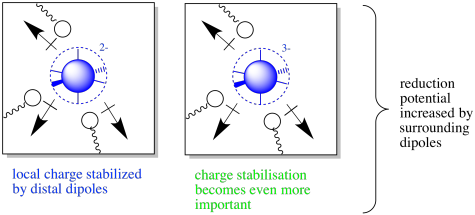
Figure MB3.5. The oxidized (left) and reduced state (right) of a coordination complex with surrounding dipoles.
Problem MB3.3.
Of the following pairs of amino acids, which one is more polar?
a) lysine or leucine b) alanine or glutamine c) histidine or valine
d) Ile or Asp e) Pro or Thr f) Asn or Ala
g) K or M h) Y or F i) R or E
Of course, it doesn't have to be an amino acid residue that interacts with the charged metal centre. The backbone of the protein itself has some very polar carbonyls. Remember, these carbonyls are especially polar because of π-donation from the amide nitrogens. One of these moieties could be oriented in such a way as to interact with a metal centre, influencing its reduction potential.
There may also be water molecules present in the interior of the protein. The coordination complex may be found in a large enough fold or cavity along the protein that water molecules are allowed to enter, stabilising any charges.
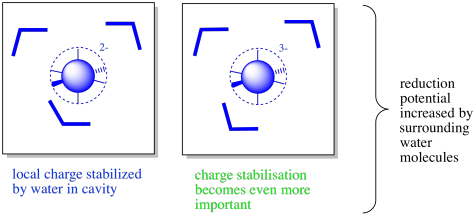
Figure MB3.6. The oxidized (left) and reduced state (right) of a coordination complex with surrounding water molecules.
Furthermore, conformational changes upon oxidation or reduction may lead to the opening or closing of channels into the protein. Conformational changes could be linked to the redox activity because metal-ligand bond may lengthen or contract as a result of oxidation-reduction reactions. Consequently, it is possible that waters would be present in one state but not in another. From a predictive point of view, this makes for an extremely complicated situation. However, from the point of view of nature, it provides for an enhanced level of control over the properties of the metalloprotein.
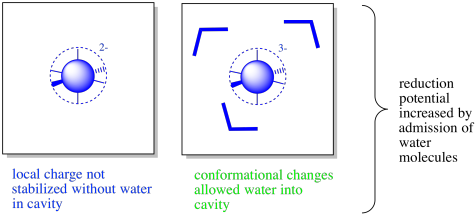
Figure MB3.7. The oxidized (left) and reduced state (right) of a coordination complex, surrounded by water molecules only in the reduced state.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: