CO3. General Reactivity Patterns, Part A
In the following pictures, a number of anions are added to a simple carbonyl compound, a ketone (2-propanone, or acetone). In each case, addition of the nucleophile is followed by addition of a proton source. Note that, overall, the reaction involves addition of the nucleophile to the carbonyl carbon and addition of the proton to the carbonyl oxygen.
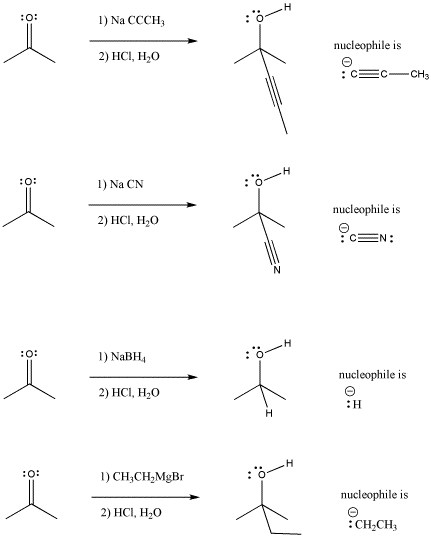
Figure CO3.1. Figure CO3.1. Addition of some anionic (and "semi-anionic") nucleophiles to a ketone.
-
Addition of anionic nucleophiles to ketones or aldehydes transforms the carbonyl into an alcohol.
Look at the way the reaction is presented in each case. The organic (carbon-based) starting material is presented on the left hand side of the reaction arrow. The reagent added to this starting material is often shown over the arrow. This reagent transforms the starting material into something else. That something else, the product, is shown to the right of the arrow.
Very often, the solvent for the reaction is shown underneath the arrow. The solvent is the liquid that is used to dissolve the starting material and reagents. This is done for a number of reasons. First, reactions generally happen much more quickly in solution than they do without a solvent. When dissolved, the reactants can move around more easily and bump into each other, as if they are swimming. Also, most useful reactions generate heat, and the solvent acts as a heat sink, carrying the excess heat away. (People who have not thought about the importance of solvent sometimes accidentally start fires as a result.) However, there are exceptions, and not all reactions need solvent.
These reactions shown above do need solvent, but the solvent is not shown for other reasons. There is something else to focus on, and the solvent would have just cluttered up the picture. Instead, the focus of the picture is that these reagents must be added in a particular order: first the nucleophile and then the acid. The nucleophile and acid cannot be allowed to mix before the nucleophile has a chance to react with the carbonyl. If they did, they would just react with each other, and leave the carbonyl electrophile alone.
Problem CO3.1.
a) For each of the cases shown above, use curved arrows to show the movement of electrons in the reaction between the anion and the carbonyl.
b) Show the intermediate that results.
c) Use curved arrows to show the movement of electrons in the reaction of the intermediate with acid to form the product.
Problem CO3.2.
a) For each of the cases shown above, used curved arrows to show what would happen if acid were mixed with the nucleophile.
b) Why would the nucleophile no longer be able to react with the carbonyl?
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carbonyl Addition Index
Back to Web Materials on Structure & Reactivity in Chemistry