CO3b. General Reactivity Patterns with Neutral Nucleophiles
Previously, we saw that nucleophiles add to carbonyl electrophiles, breaking the pi bond of the carbonyl and converting it into an OH group.
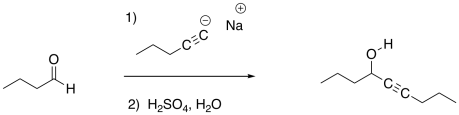
Figure CO3b.1.Addition of an anionic nucleophile to an aldehyde.
The pattern of reactivity is very different with another class of nucleophile. These could be called neutral nucleophiles (as opposed to anionic ones). Neutral nucleophiles do not have a negative charge like the previous ones. However, they still have a lone pair, and that fact still makes them nucleophiles.
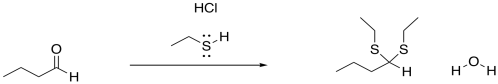
Figure CO3b.2.Addition of a neutral nucleophile to an aldehyde.
However, the outcome of the reaction looks a little different from what we saw with the earlier anionic nucleophiles. In this case, the carbonyl is not converted into an OH group. (At some point we will see that it can be under some circumstances, but that is the exception rather than the rule.) Instead, the oxygen is completely displaced from the carbonyl. It is lost as water. It is replaced by two nucleophiles, instead.
- Neutral nucleophiles can add to carbonyls, displacing the oxygen atom in the form of water.
The same thing happens in the following case, involving an oxygen nucleophile instead of a sulfur. Oxygen is in the same column of the periodic table as sulfur, so similar behaviour is not really a surprise.
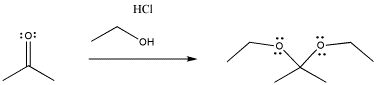
Figure CO3b.3.Addition of a neutral alcohol nucleophile to a ketone.
With nitrogen nucleophiles, the oxygen of the carbonyl is still displaced as water, but the nucleophile does not appear to add twice. Instead, the C=O is replaced with a C=N.
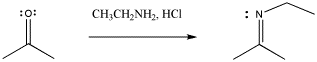
Figure CO3b.4.Addition of a primary amine nucleophile to a ketone.
Other nitrogen compounds give a similar product, with the double bond in a slightly different place. We will see that the difference has to do with how many hydrogens there are on the original nitrogen atom. If there are two, it is an even trade: the nitrogen replaces the carbonyl oxygen, and the oxygen takes the two hydrogens to form water. If there is only one NH, the oxygen needs to pick up a second hydrogen from elsewhere, and it takes it from the alpha position, next to the former carbonyl.
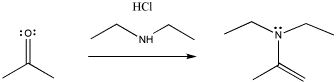
Figure CO3b.5.Addition of a secondary amine nucleophile to a ketone.
These nucleophiles are a little less likely to react with the acid, so we have not taken care to add them in a particular order. They are less likely to react with the acid because they are not anions. They are neutral, so they will not attract the proton as strongly as anions would. In reality, you have to be a little bit careful about conducting reactions like these. With the amine nucleophiles in particular, if you add too much acid, the nucleophiles will react with the acid after all.
Take another look at the general pattern of reactivity for the anionic nucleophiles and the neutral nucleophiles. In the case of the anionic nucleophiles, the pattern is relatively easy to discover. The product has incorporated the nucleophile into its structure (or at least the anionic part of the nucleophile, which you will soon learn about). The nucleophile has attached at the carbonyl carbon. The carbonyl oxygen has become part of a hydroxyl group. These are very common patterns in the addition of nucleophiles to carbonyls.
In the case of the neutral nucleophiles, there are some similarities and some differences. The nucleophile is still incorporated into the product structure. It has added at the carbonyl position in the electrophile. However, the fate of the carbonyl oxygen is a little bit different with neutral nucleophiles. Generally, this atom is lost as a water molecule in these cases. If you look closely, you will be able to tell where the two protons come from in each case in order to form the water molecule. It's not really the HCl, which is only added in very tiny amounts and acts catalytically. The protons come from other positions in the nucleophile, and sometimes from the electrophile, too.
- These neutral nucleophiles generally have a proton attached to the nucleophilic atom.
- These protons become part of the water molecule made when the carbonyl oxygen is lost.
This chapter will help you to develop skills so that you can recognize where nucleophilic additions have taken place in reactions. You will also be able to predict what products may result from a nucleophilic addition.
