CO22b. Michael Addition and Robinson Annulation
The Michael addition is one of the most important examples of conjugate addition. In a Michael addition, an enolate nucleophile undergoes 1.4-addition to an enone. Typically, the nucleophile is doubly activated; that is, there is a carbonyl on either side of the alpha position.
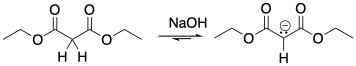
Figure CO22b.1. A soft, conjugated Michael nucleophile.
- The nucleophile for a Michael addition is a doubly-activated enolate ion.
- A doubly-activated enolate precursor has a carbonyl or similar pi electron-withdrawing structure on either side of a CH2 group.
In a Michael addition, the nucelophile and electrophile are often added together as neutral species. The nucleophile is activated by deprotonation to make the anion. That can be done using a strong bas such as NaOH or NaOCH2CH3. It's okay if there isn't complete deprotonation because the reaction can proceed all the way under equilibrium. As in other conjugate additions, the addition of the nucleophile results in placement of a negative charge in the α-position, next to the carbonyl. The reaction essentially results in formation of a relatively stable enolate ion. The enolate ion is still fairly basic, and it can take a proton from the alcohol solvent to make the neutral ketone product.

Figure CO22b.2. A Michael addition reaction.
- In a Michael addition, a doubly-activated enolate undergoes conjugate addition or 1,4-addition to an α,β-unsaturated carbonyl.
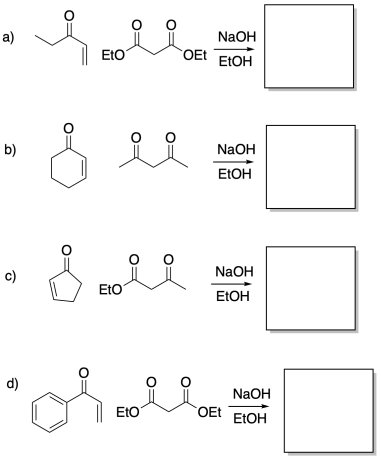
Problem CO22b.7.
Provide the product of the following Michael additions.
Robinson Annulation
Robinson annnulation allows for formation of a six-membered ring via a 2-step sequence: a Michael addition and subsequent aldol condensation. The reactions proceed automatically, one after the other. The reaction was developed by Sir Robert Robinson as he ressearched the chemistry of alkaloids. Robinson later received the Nobel Prize.
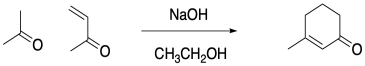
Figure CO22b.3. A Robinson annulation.
- A Robinson annulation forms a 6-membered ring from two molecules: a ketone and a second, α,β-unsaturated ketone.
The first reaction, a Michael addition, links two molecules together via a 1,4-addition of an enolate to an enone. The doubly activated nucleophile is deprotonated in the α-position. It then adds to the 1,4-position of the enone.
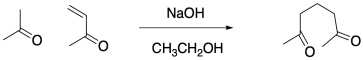
Figure CO22b.4. The Michael step in a Robinson annulation.
- The first step in a Robinson annulation is a Michael addition.
After that, a regular aldol reaction ensues, closing the ring. Another α-position gets deprotonated. That enolate adds directly to a C=O in a 1,2-addition. There are sometimes different possible combinations here, but generally a six-membered ring results.
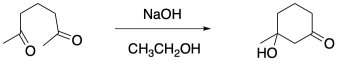
Figure CO22b.5. The aldol step in a Robinson annulation.
Typically, the aldol reaction continues through a condensation. That's the variation of the aldol reaction in which a water molecule is lost, forming a double bond in conjugation with the carbonyl.
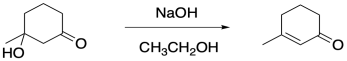
Figure CO22b.6. The condensation step in a Robinson annulation.
- The second step in a Robinson annulation is an intramolecular aldol condensation.
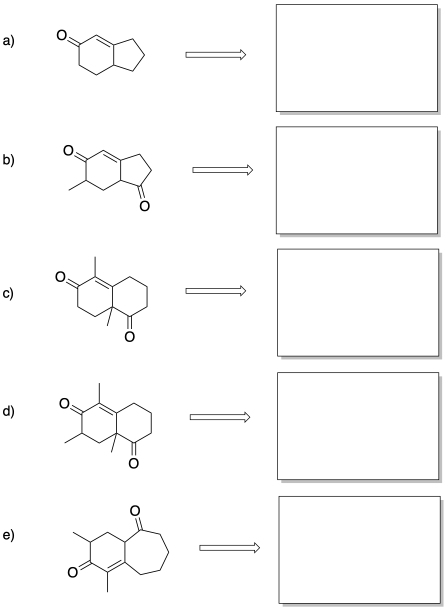
Problem CO22b.8.
Provide mechanism for the following steps in the Robinson annulation above:
a) the Michael addition
b) the aldol reaction to form the six membered ring
c) the dehydration of the aldol product to form the enone product
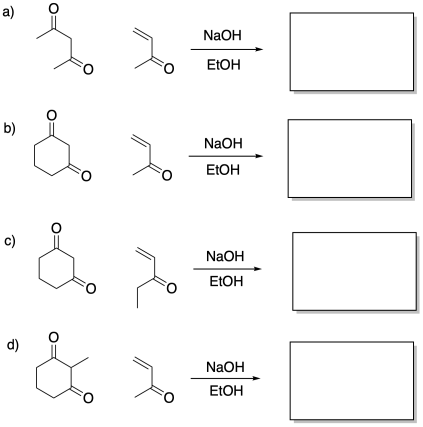
Problem CO22b.9.
Provide products of the following Robinson annulations.
Sometimes, it is useful to think "backwards" about a reaction. Given a particular structure, can we picture the reaction that may have taken place to form that structure? This type of analysis is very useful to bio-organic chemists, who often seek to find out how different compounds formed in nature. By imagining different ways in which a natural product may have formed, they can design different experiments that may shed light on how the process really happened.
Of course, synthetic chemists find this way of thinking about things is very helpful, too. Given the task of making a particular compound, they must imagine the most efficient ways in which the compound could be made.
Let's take a look at how that
approach to thinking about reactions would work with a Robinson Annulation.
There are two reactions in a Robinson Annulation. One is a Michael addition and
another is an aldol condensation. They always happen in that order. To analyse
the product, we need to work backward from the product and "disconnect" the
molecule. That means we need to find where the aldol reaction happened, then
where the Michael reaction happened.
A diagnostic aldol fragment can look
either like R-CO-CH2-CHOH-R (carbonyl-carbon-alcohol) or, if there is
a condensation/dehydration step, RCO-CH=CHR (carbonyl-alkene). That's the first
part of the molecule you need to find. It forms when the enolate fragment RCO-CH2-
adds to the carbonyl RCHO. We must break the product at the double bond to
uncover the aldol reactant.
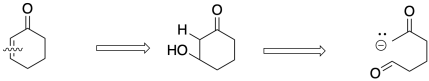
Figure CO22b.7. Retrosynthetic analysis of the aldol condensation step in a Robinson annulation.
A Michael fragment looks like R-CO-CH2-CH2-CH2-CO-R. There are two carbonyls, with three carbons in between them. That's the part of the molecule we need to find next, but we won't see it until we have stepped back to the situation we had before the aldol step. The Michael fragment forms from the electrophile RCO-CH=CH2 and the enolate nucleophile -CH2(CO)R.

Figure CO22b.8. Retrosynthetic analysis of the Michael step in a Robinson annulation.
Overall, the Robinson annulation is the sum of these two steps.
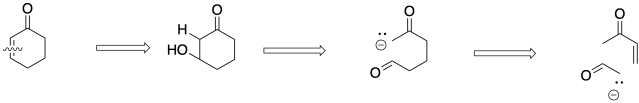
Figure CO22b.9. Overall retrosynthetic analysis of a Robinson annulation.