CO6. Relative Reactivity of Carbonyls
There are two kinds of electrophiles that commonly undergo nucleophilic addition at the carbonyl group: aldehydes and ketones. Of these, aldehydes are a little more reactive. Let's look at some factors in the reactivity of carbonyls.
Sterics
Steric hindrance, or crowdedness around the electrophile, is an important factor that influences reactivity. Aldehydes and ketones are very similar, but because an aldehyde has a little hydrogen atom attached to the carbonyl carbon, the carbonyl is much less crowded in an aldehyde than in a ketone. That makes it much easier for a nucleophile to interact with an aldehyde carbonyl than a ketone carbonyl.
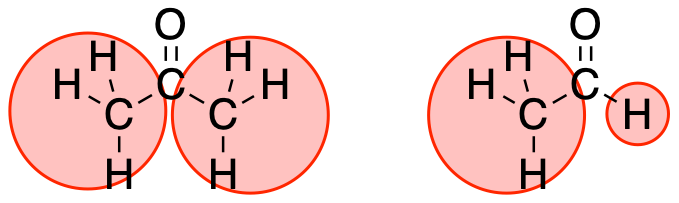
Figure CO6.1. Crowding in a ketone compared to an aldehyde.
- The less crowded the electrophile, the more easily it will react.
- Aldehydes are more reactive than ketones.
Crowdedness affects reactivity simply by preventing nucleophiles from easily approaching the electrophilic site in the carbonyl. If the nucleophile hits something other than the carbonyl carbon, it will probably just bounce off. It needs to collide with the carbonyl carbon in order to deliver its electrons to the right place.
Charge
Amount of positive charge on the electrophile is an important factor that influences reactivity.
- The more positive the electrophile, the more easily it will react.
Factors that place more positive charge on the carbonyl (electron withdrawing groups nearby) make the carbonyl more positive and more reactive. Factors that place additional electron density on the carbonyl (electron donors nearby) make the carbonyl less reactive.
There is another resonance structure that we can think about that illustrates the electrophilicity of a carbonyl. That structure places a full negative charge on the oxygen and a full positive charge on the carbon. This isn't a good Lewis structure because the carbon does not have an octet. Nevertheless, when taken together with the regular Lewis structure, it suggests something real about the nature of the carbonyl: there is partial positive charge on the carbon and partial negative charge on the oxygen.
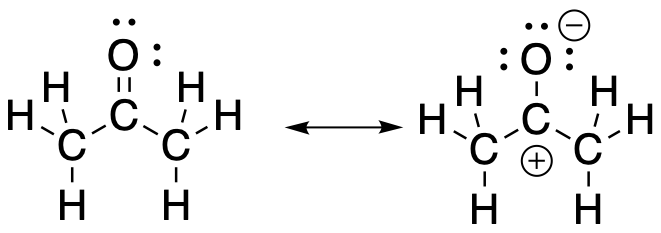
Figure CO6.2. Two resonance structures of a carbonyl.
There is a general rule about cation stability on carbon atoms: a carbocation with more carbons attached to it is more stable than a carbocation with more hydrogens attached to it.
This observation is sometimes explained as an inductive effect. The positively charged carbon is more electronegative than the uncharged carbons, so it draws electrons away from them. It can polarize the neighbouring carbons, drawing some negative charge towards itself and leaving some positive charge on the other carbons. In that way, it s charge is delocalized and stabilized.
In a more sophisticated explanation, the cation becomes stabilized by a molecular orbital interaction involving the empty p orbital on the carbocation and C-H bonds on the neighbouring carbons.
A similar situation results in the partially positive carbon in the carbonyl. The carbonyl carbon in the ketone is a little more stable than the carbonyl carbon in the aldehyde.
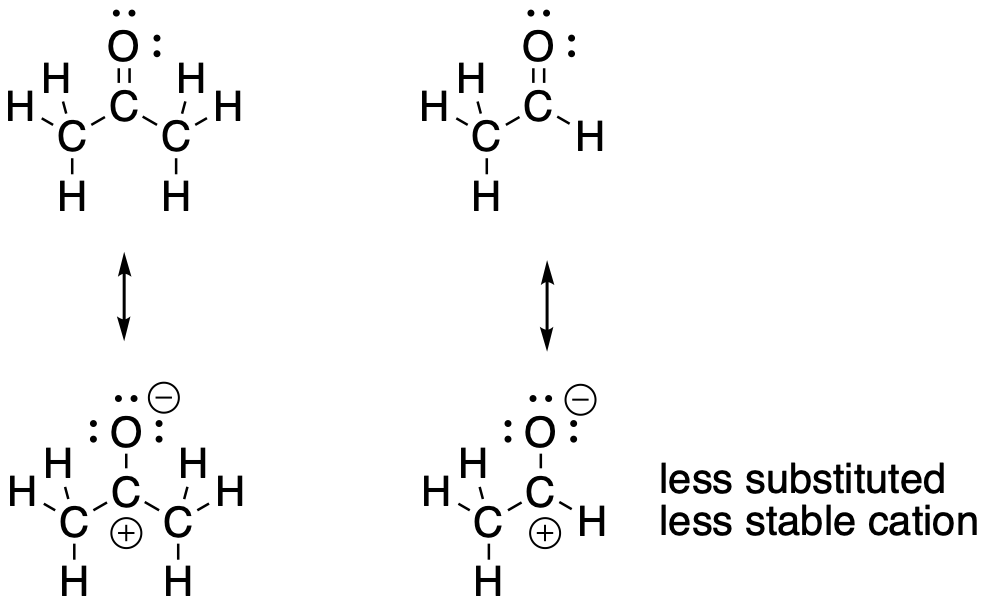
Figure CO6.3. Cation stability in a ketone compared to an aldehyde.
- The partial positive charge on an aldehyde carbonyl carbon is less stable than the partial positive charge on a ketone carbonyl carbon.
- Again, aldehydes are more reactive than ketones.
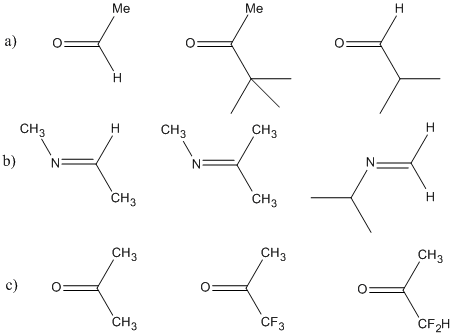
Problem CO6.1.
Rank the following carbonyl compounds from most reactive to least reactive towards nucleophilic addition. Explain your reasoning.
Problem CO6.2.
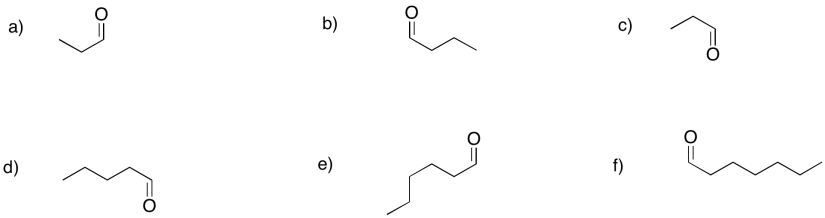
Provide names for the following aldehydes.
Problem CO6.3.
Provide names for the following ketones.
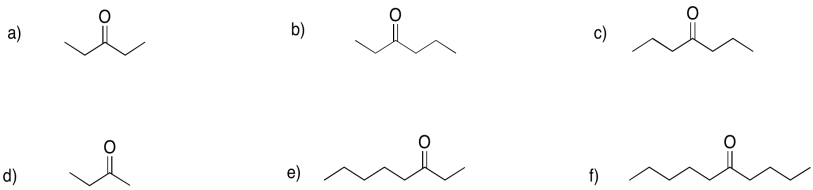
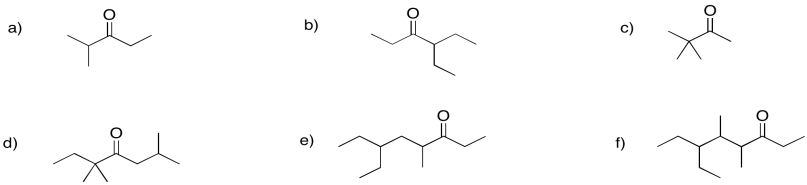
Problem CO6.4.
Provide names for the following ketones.