CO27. Solutions to Selected Problems, Part A
Problem CO1.1.
The HO-C=O or CO2H group present in all of the amino acids is called a carboxylic acid.
An additional carboxylic acid is present in aspartic acid and glutamic acid.
The H2N-C=O or CONH2 group present in asparagine and glutamine is called an amide.
Problem CO1.2.
a) aldehyde b) ketone c) ketone d) aldehyde
Problem CO1.3.
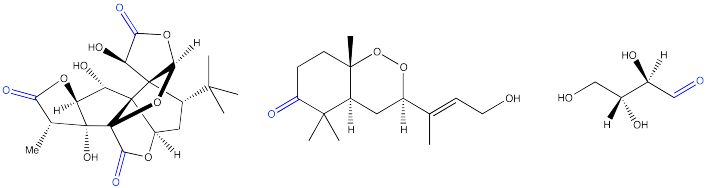
Problem CO1.4.
Problem CO2.1.
a) The double bond means two pairs of electrons are shared between the carbon and oxygen, instead of just one. As a result, the oxygen is able to pull more electron density away from the carbon. The carbon becomes much more positive in this case than in the case of a carbon-oxygen single bond.
Not only that, but the second bond between the carbon and the oxygen is a pi bond. Those electrons are farther from the nucleus than a sigma bond, in which the electrons are tightly held between the atoms. That means the pi electrons are more easily drawn toward the oxygen, so the bond becomes even more polarized.
b) A C=N bond would be very similar to a C=O bond, because nitrogen is the third most electronegative element after oxygen and fluorine (ignoring noble gases).
Problem CO2.2.
Problem CO2.3.
Problem CO2.4.
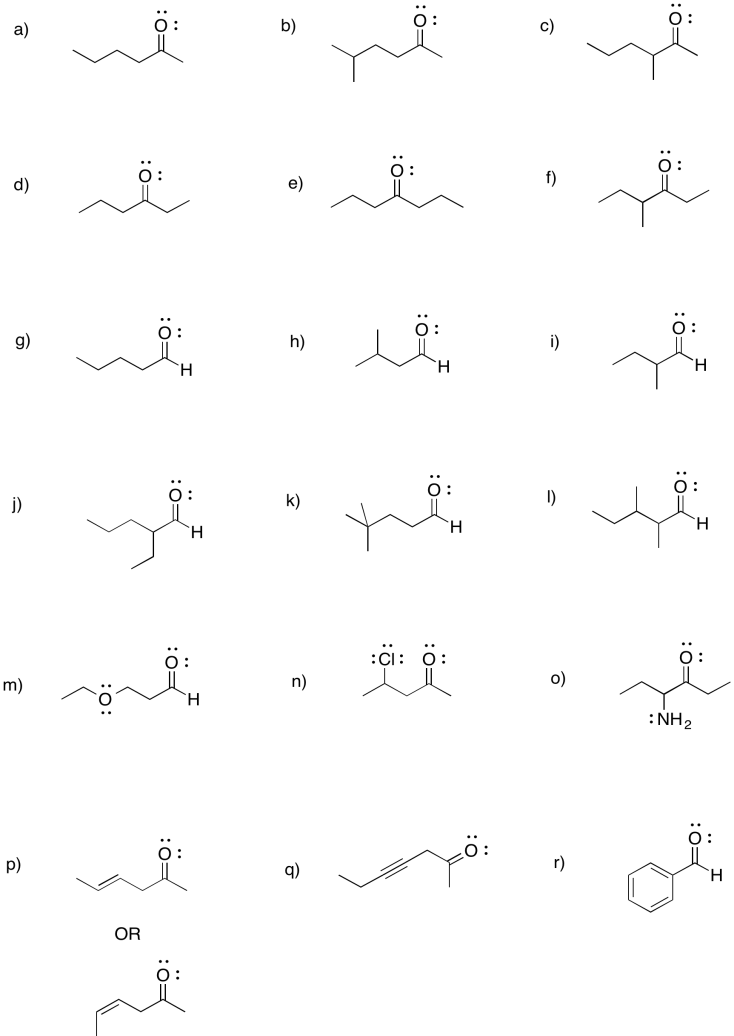
Problem CO2.5.
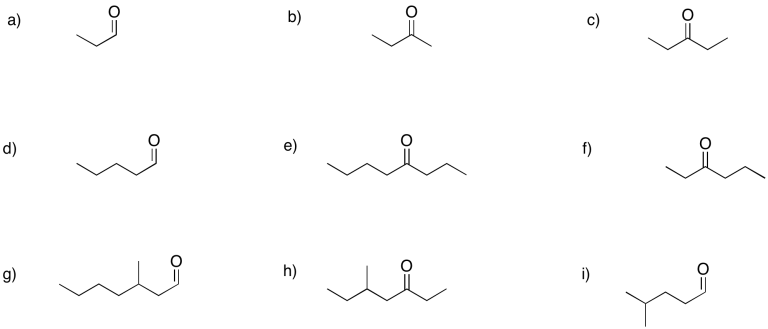
a) 2-hexanone or hexan-2-one b) 5-methylhexan-2-one c) 3-methylhexan-2-one
d) hexan-3-one e) heptan-4-one f) 4-methylhexan-3-one
g) pentanal h) 3-methylbutanal i) 2-methylbutanal
j) 2-ethylpentanal k) 4,4-dimethylpentanal l) 2,3-dimethylpentanal
m) 3-ethoxypropanal n) 4-chloropentan-2-one o) 4-aminohexan-3-one
p) hex-4-en-2-one (in the solution above, (E)-hex-4-en-2-one is shown first and (Z)-hex-4-en-2-one is shown second).
q) hept-4-yn-2-one r) benzaldehyde (this is a common name adopted for formal naming. Benz means a carbon attached to a benzene ring.)
Problem CO2.6.
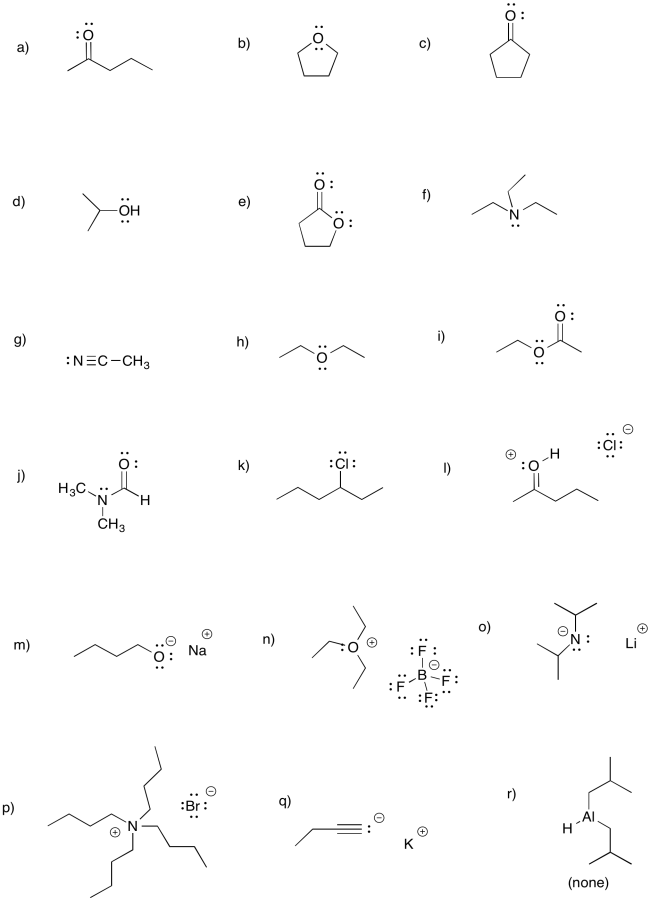
Problem CO3.1.
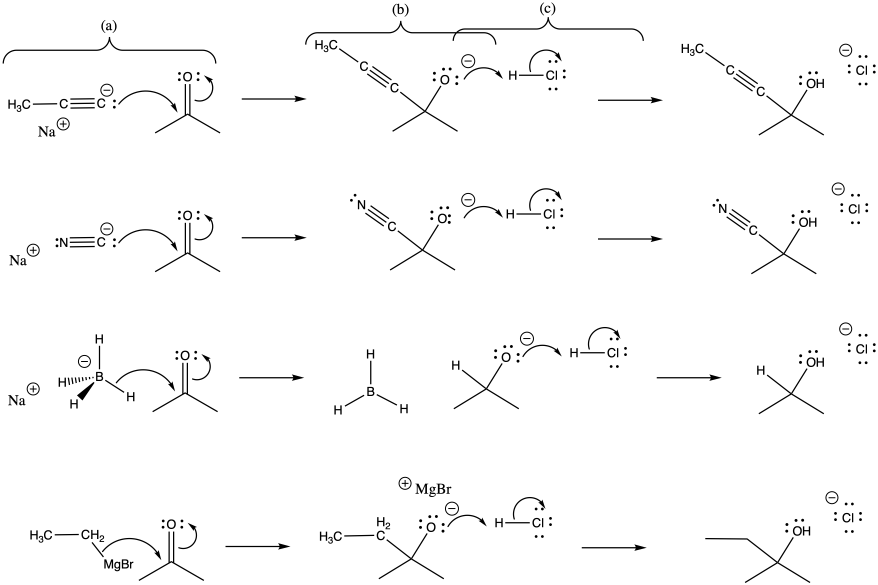
Problem CO3.2.
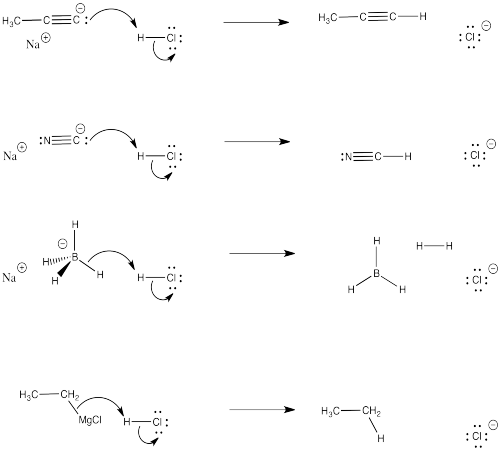
Problem CO4.1.
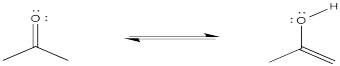
Problem CO4.2.
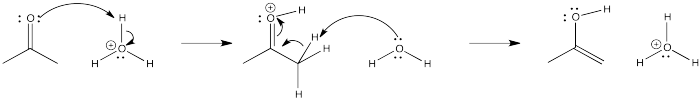
Problem CO4.3.
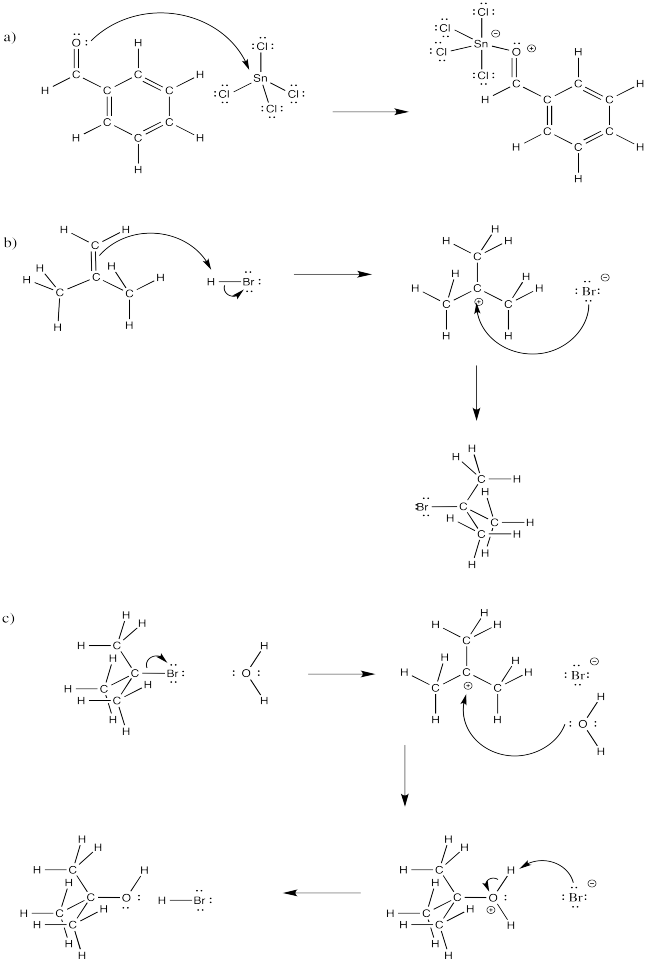
Problem CO4.4.
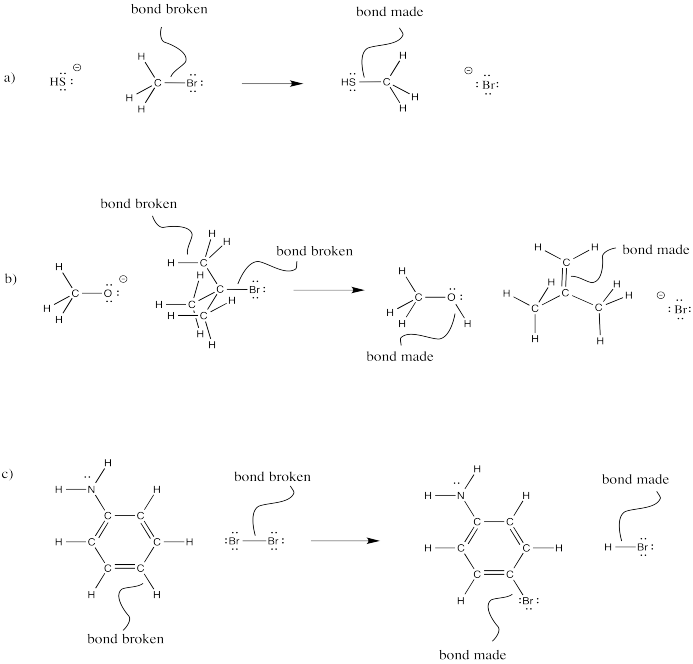
Problem CO4.5.
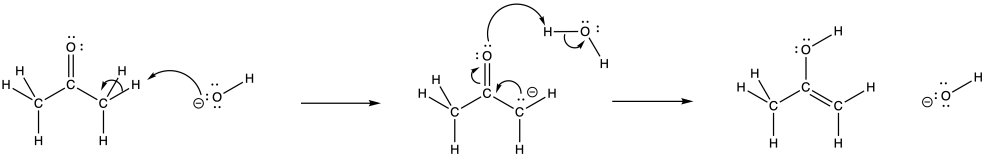
Problem CO5.1.
The LUMO in this case is the π*, an antibonding level. If electrons populate this level, the π bond will break.
Problem CO5.2.
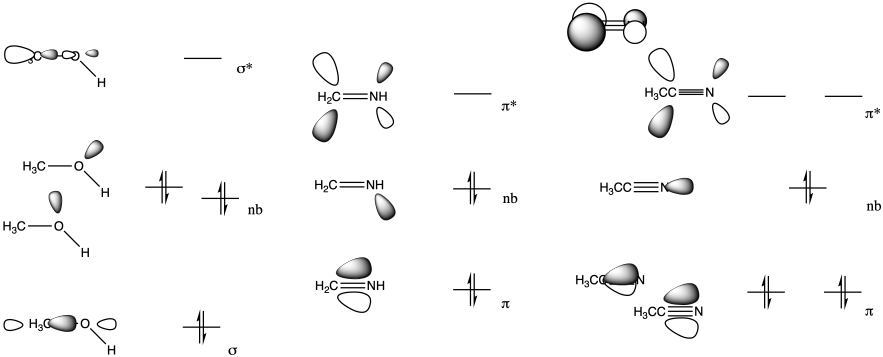
Problem CO5.3.
Both the imine (b) and nitrile (c) have a low-lying pi antibonding level (π*), similar to a carbonyl.
Problem CO6.1.
a) On the basis of steric crowding, the first one is most reactive, then the last one, then the middle.
b) On the basis of steric crowding, the last one is most reactive, then the first one, and then the middle.
Although there is a large group on the nitrogen in that last compound, the site of reactivity is the carbon, which is less crowded.
c) On the basis of electronics, the middle one is most reactive, then the last, and then the first. The fluorine atom is very electronegative and pulls electron density towards itself. That leaves more positive charge on the nearby carbonyl carbon. The more fluorines on that nearby carbon, the more positive the carbon. The more positive the carbon, the more it attracts electrons from a nucleophile.
Problem CO6.2.
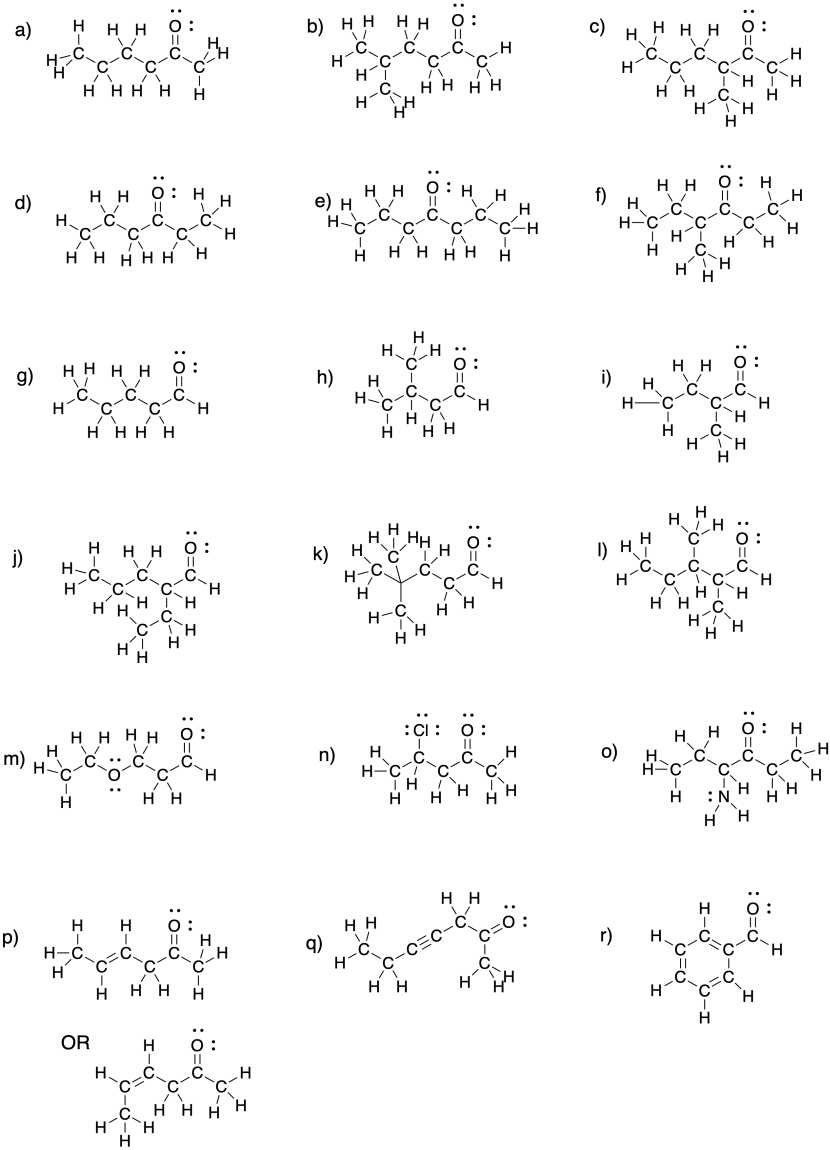
a) propanal b) butanal c) propanal, again!
d) pentanal e) hexanal f) heptanal
Problem 6.3.
a) 3-pentanone b) 3-hexanone c) 4-heptanone
d) 2-butanone e) 3-octanone f) 5-decanone
Problem 6.4.
a) 2-methyl-3-pentanone or 2-methylpentan-3-one b) 4-ethyl-3-hexanone or 4-ethylhexan-3-one
c) 3,3-dimethyl-2-butanone or 3,3-dimethylbutan-2-one d) 2,5,5-trimethyl-4-heptanone or 2,5,5-trimethylheptan-4-one
e) 6-ethyl-4-methyl-3-octanone or 6-ethyl-4-methyloctan-3-one f) 6-ethyl-4,5-dimethyl-3-octanone or 6-ethyl-4,5-dimethyloctan-3-one
Problem CO7.1.
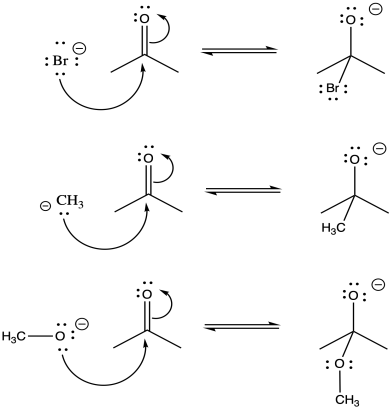
With bromide anion, there is a stable, polarizable anion on the left. The alkoxide ion on the right experiences lone pair-lone pair repulsion. Overall, equilibrium lies heavily to the left.
With methyl anion, there is a less stable anion on a relatively low-electronegativity carbon on the left compared to the more electronegative oxygen on the right. In the absence of any lone pair-lone pair repulsion on the right, this equilibrium lies heavily to the right.
There is a moderately stable alkoxide anion on both side. The lone pair-lone pair repulsion on the right pushes the equlibrium appreciably to the left, although not entirely; some of the species on the right would still be present at equilibrium.
Problem CO8.1.
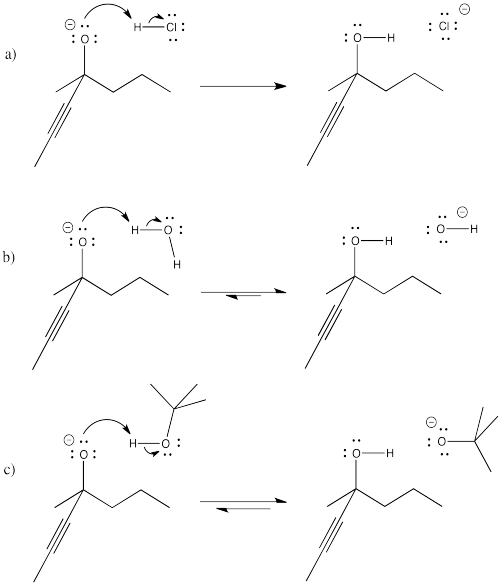
Problem CO8.2.
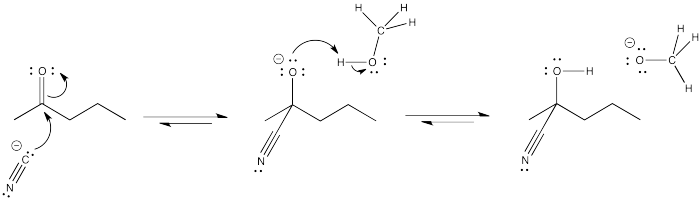
The high concentration of methanol when it is used as a solvent pushes the equilibrium to the right in the last step, based on le Chatelier's principle.
Problem CO8.3.
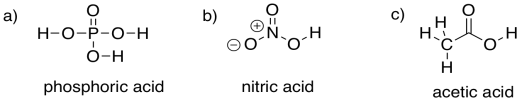
Problem CO8.4.
Ammonium chloride has an N-H bond, normally less polar than the O-H bond of water. However, the positive charge makes this compound give up a proton more easily, because it results in a neutral (uncharged) ammonia molecule.
Sodium carbonate has a polar O-H bond, just like water. However, the anion that results from loss of a proton is resonance-stabilised. That makes this compound more acidic than water.
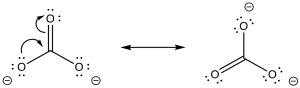
Problem CO8.5.
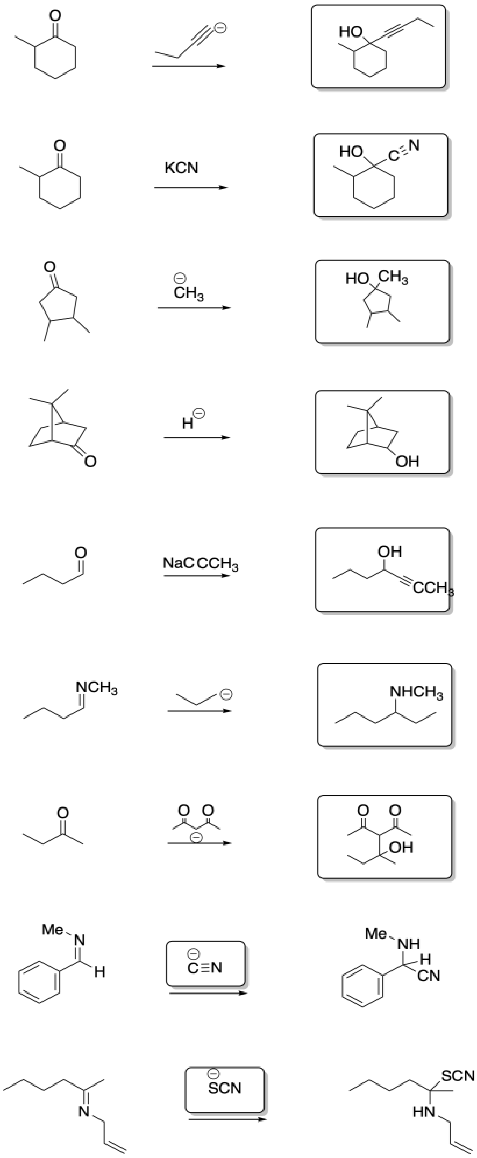
Problem CO8.6.
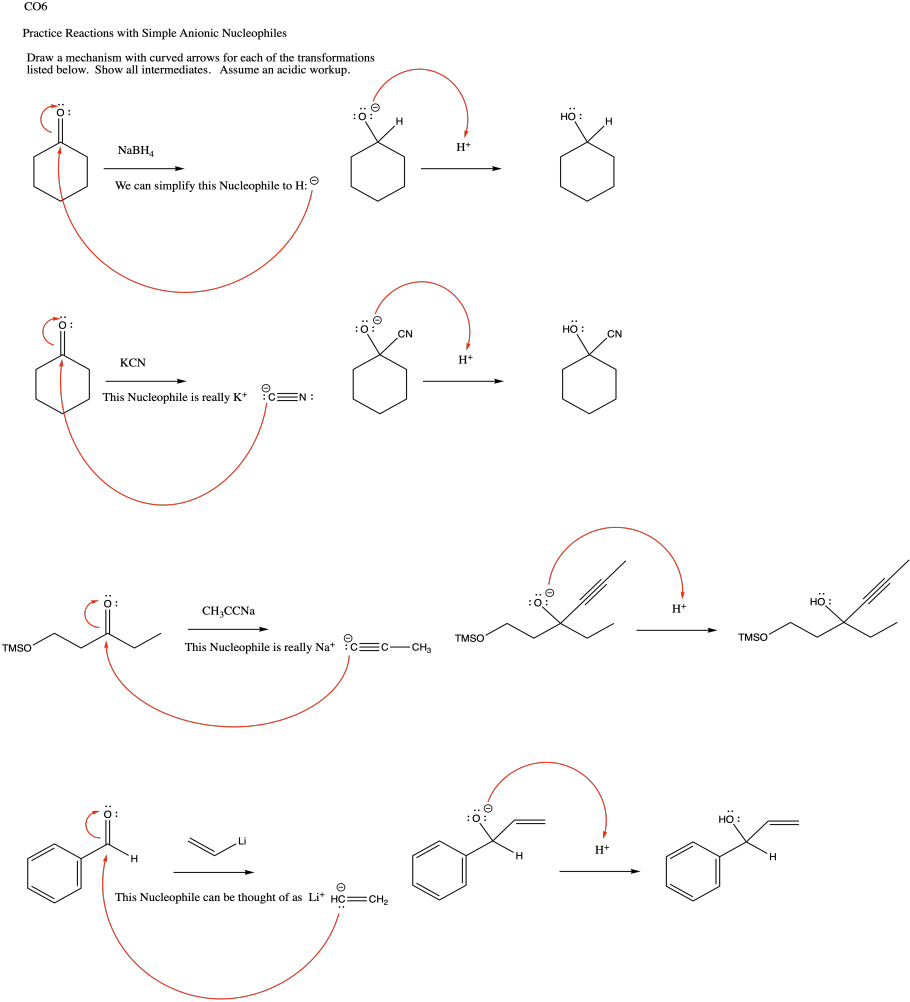
Problem CO9.1.
a) In acetylide, the lone pair is on a linear carbon or sp carbon. In methyl anion, the lone pair is on a tetrahedral carbon or sp3 carbon. The description "sp" indicates that sigma bonding to neighbours involves a 2s orbital and a 2p orbital on carbon; there is a 50% contribution from the s orbital.
The description "sp3", on the other hand, indicates that sigma bonding to neighbours involves a 2s orbital and three 2p orbitals; there is a 25% contribution from the s orbital.
The 2s orbital is lower in energy than the 2p orbital. The greater the s orbital contribution to the bond (or in this case to the lone pair), the lower it is in energy. Thus, a lone pair on an sp carbon is lower in enrgy than a lone pair on an sp3 carbon.
b) In cyanide, the same argument outlined in pary (a) hold true. In addition, the nearby electronegative nitrogen stabilizes the charge by drawing electron density toward itself.
Problem CO9.2.
a) CH3OK, because of the ionic O-K bond. This is an anionic nucleophile. It is more reactive and nucleophilic than the corresponding neutral nucleophile.
b) CH3NH2, because nitrogen is less electronegative than oxygen. Its lone pair is held less tightly and is more easily donated to the electrophile.
c) NaCCH, because the neighbouring carbon in this case does not have the inductive electron-withdrawing effect that the nitrogen does in the case of NaCN. In that case, the lone pair is stabilized and made less reactive.
d) c-C6H11ONa, because the negative charge is localized on one atom. In c-C6H5ONa, the negative charge is delocalized over four different positions in the molecule. Delocalization of charge stabilizes the anion and makes it less reactive.
Problem CO9.3.
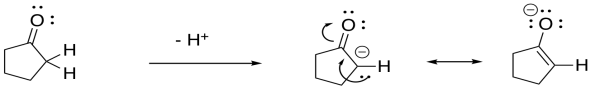
The resulting anion is stabilised by resonance.
Problem CO9.4.
The case with more steric crowding is more likely to result in deprotonation.
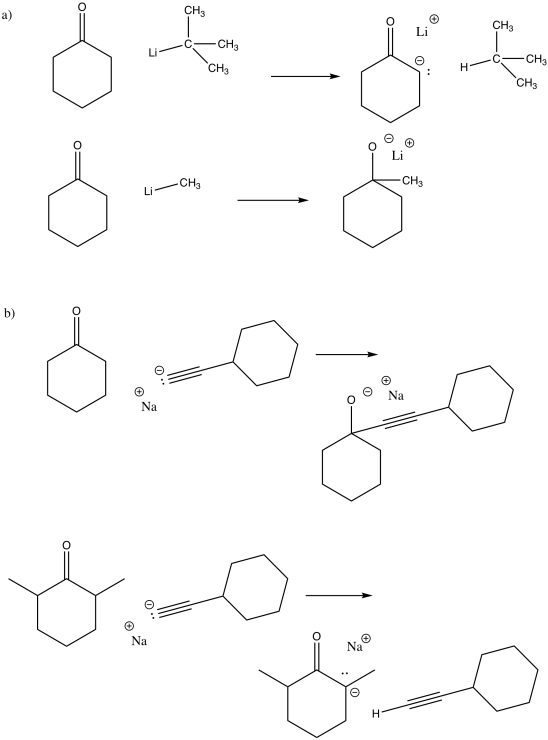
Problem CO11.1.
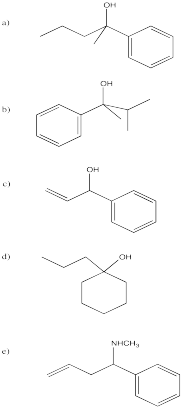
Problem CO11.2.
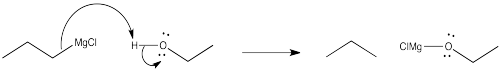
Problem CO11.3.
a)
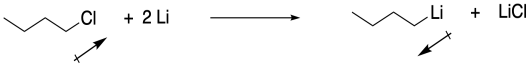
b) The direction of the bond dipole changes. Electrons are pulled more toward chlorine than carbon, but more toward carbon than chlorine. The carbon was not nucleophilic in the chlorobutane, but it is nucleophilic in butyllithium.
c) The Pauling electronegativity value of lithium is 0.98; it is 1.31 in magnesium. In both cases, the electronegativity is considerably lower than in carbon (= 2.55). Consequently, both a carbon-lithium bond and a carbon-magnesium bond, although substantially covalent, do have a significant amount of negative charge on the carbon, making it act like a carbon with a lone pair.
Problem CO11.4.
a)
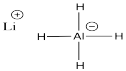
b) The hydrogen atom has considerable negative charge in AlH4- because the Pauling electronegativity value of aluminum is 1.61 compared to 2.2 in hydrogen. This covalent bond is polarized with the negative charge on hydrogen and positive on aluminum (in contrast to the formal charge, a separate idea that indicates there is more electron density at aluminum here than there would be if aluminum had no octet).
c) The bond dipole between aluminum and hydrogen is much larger in AlH4- than in BH4- because the Pauling electronegativity value of aluminum is 1.61 compared to 2.04 in boron. The electronegativity difference is much greater with aluminum than with boron.

d)
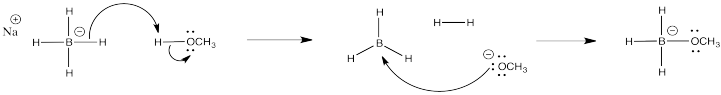
e) Students might point to the statistical factor of having only three hydrogens in NaBH3OCH3 rather than four in NaBH4, which rationally might lead to a 25% in reactivity in the former. However, the reactivity difference is much greater than that. It comes from the electron withdrawing effect of the strongly electronegative oxygen atom, which strongly attenuates the amount of negative charge on the hydrogens.
f) For reasons outlined above, the hydrogens in LiAlH4 are much more basic than those in NaBH4, so that all of the hydrogens would continue to react with the protons of water.
Problem CO11.5.
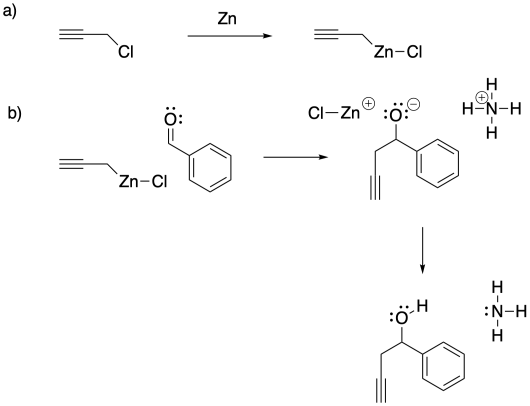
c) The Pauling electronegativity value of zinc is 1.65; it is 1.31 in magnesium. In both cases, the electronegativity is lower than in carbon (= 2.55). Consequently, both a carbon-zinc bond and a carbon-magnesium bond have a significant amount of negative charge on the carbon, making it act like a carbon with a lone pair. However, the smaller charge separation in the carbon-zinc bond leaves it much more covalent and much less susceptible to protonation by water than the carbon-magnesium bond.
d) Because the Barbier reaction can be carried out in water, it limits the need to use organic solvents. In many cases, the use of water as a reaction solvent can have fewer environmental ramifications as a result.
Problem CO11.6.
a) ThePauling electronegativity value of zinc is 1.65; it is 1.5 in tantalumum. In both cases, the electronegativity is lower than in chlorine (= 3.16). Consequently, both a chlorine-zinc bond and a chlorine-tantalum bond have a significant amount of negative charge on the chlorine and a significant amount of positive charge on the metal.
b) Zinc chloride, ZnCl2
c) You would use a 3:2 ratio of dimethylzinc to tantalum pentachloride:
3 Me2Zn + 2 TaCl5 → 2 Me3TaCl2 + 3 ZnCl2
d)
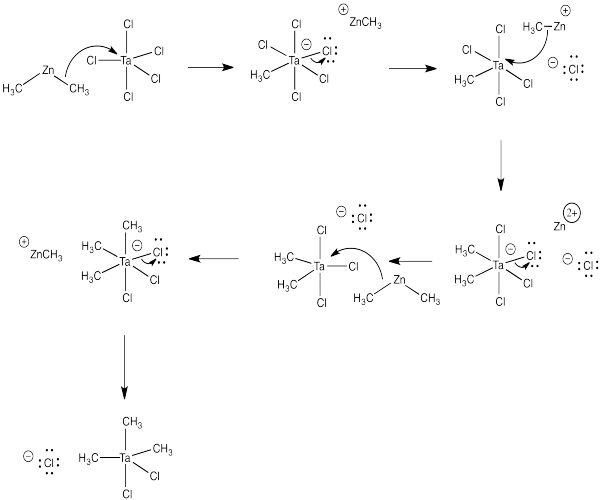
e) Don't try this at home.
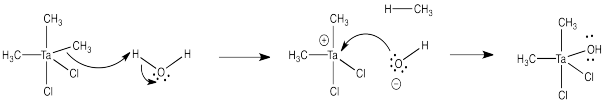
Problem CO11.7.
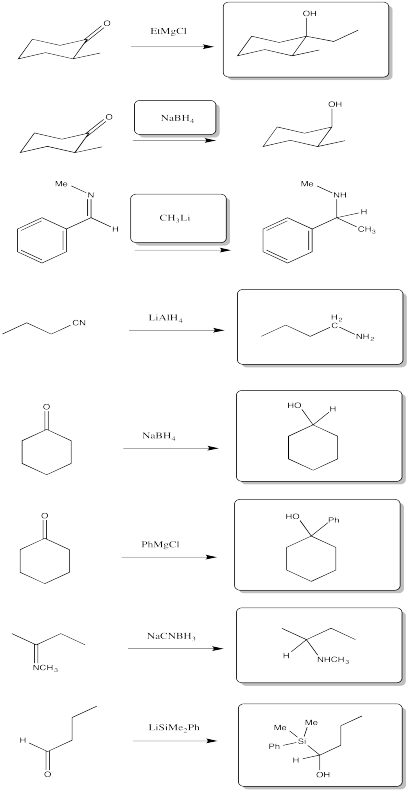
Problem CO11.8.
a) 1-pentanol b) 2-butanol c) 4-octanol (remember to number from the end that gives the lowest number)
d) 2-methylpropan-2-ol e) 3-methylhexan-2-ol f) 5,6-dimethylheptan-1-ol
Problem CO11.9.
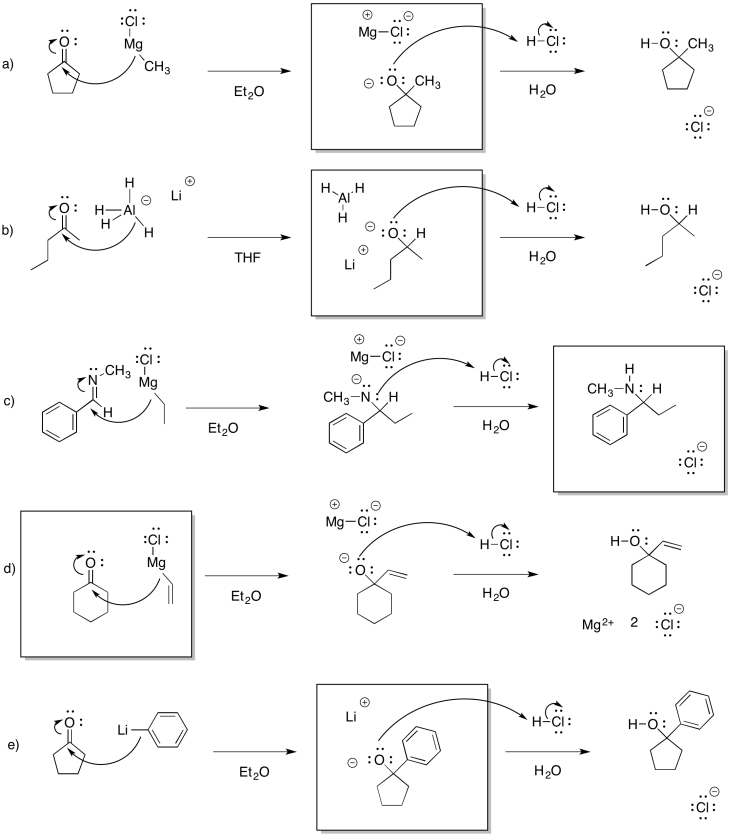
Problem CO12a.1.

Problem CO12a.2.
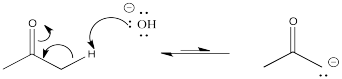
Problem CO12a.3.
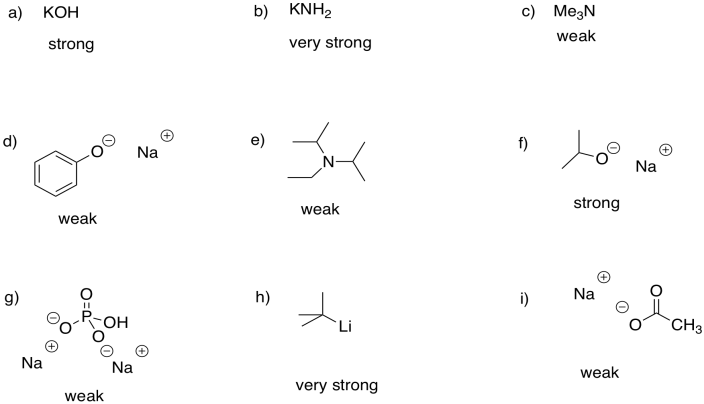
Problem CO12b.1.
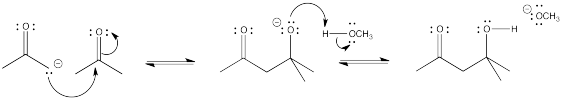
Problem CO12b.2.
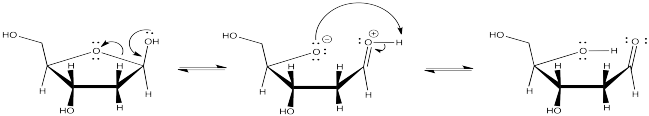
The base shown here is an aspartate amino acid residue in its deprotonated state. It's a common base in biochemical reactions.
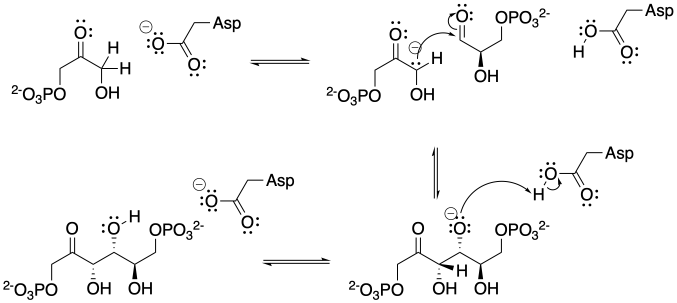
Problem CO12b.3.
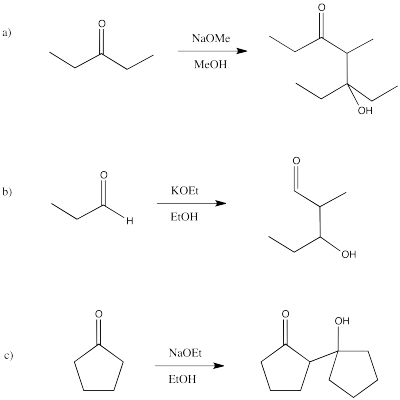
Problem CO12b.4.
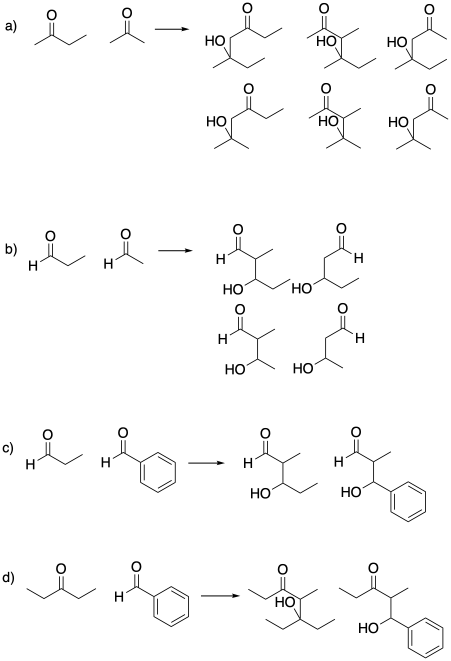
Problem CO12b.5.
Some of these compounds do not have alpha-protons, so they cannot form enolate ions.
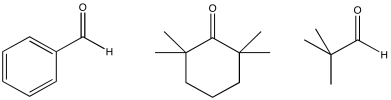
Problem CO12b.6.
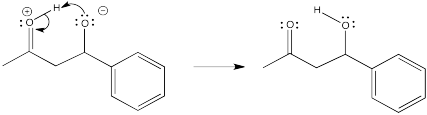
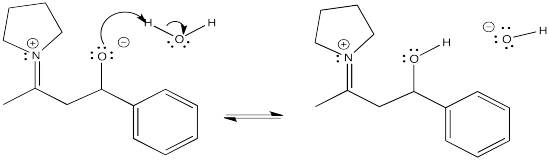
Problem CO12b.7.
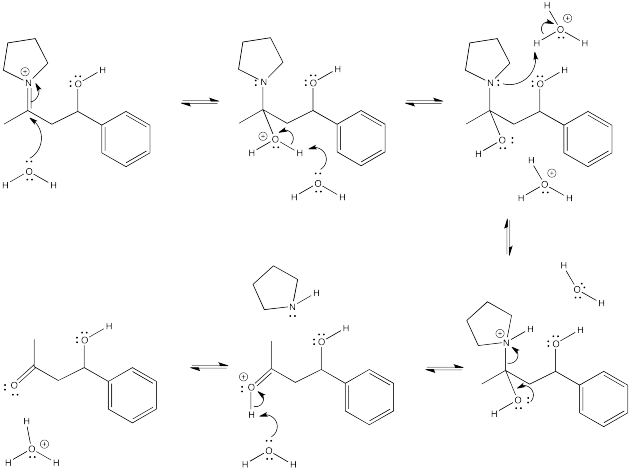
Problem CO12b.8.
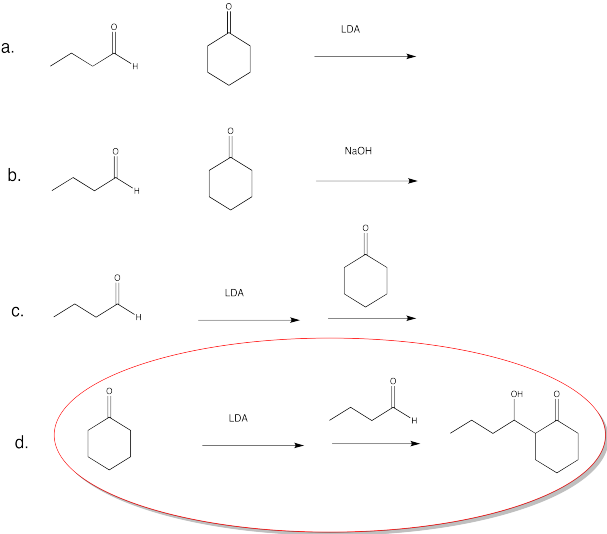
Problem CO12b.9.
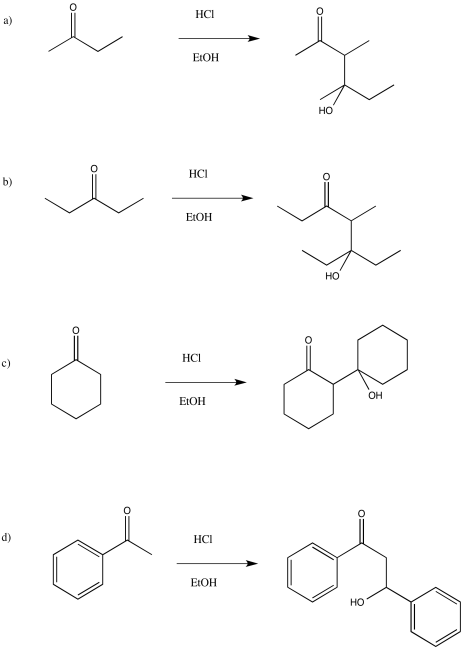
Problem CO12b.10.
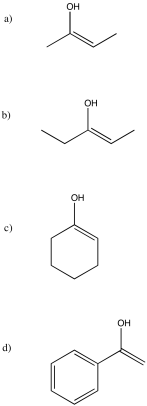
Note that, in problem (a), two different enols are possible. The one shown predominates because more-substituted double bonds are more stable than less-substituted double bonds.
Problem CO12b.11.
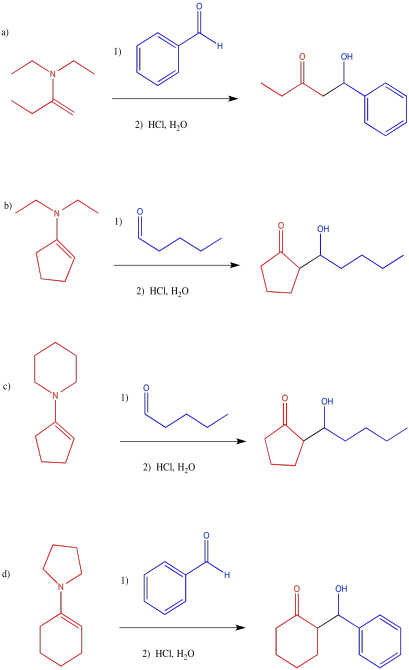
Problem CO12b.12.
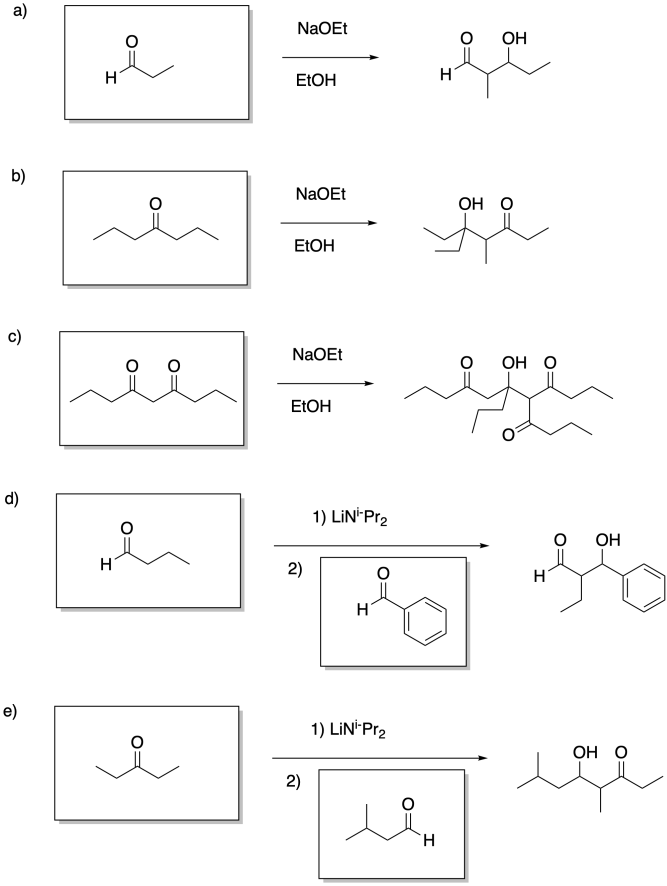
Problem CO12b.13.
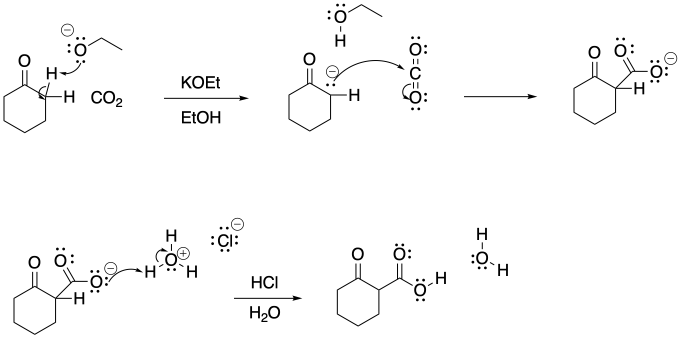
Problem CO12b.14.
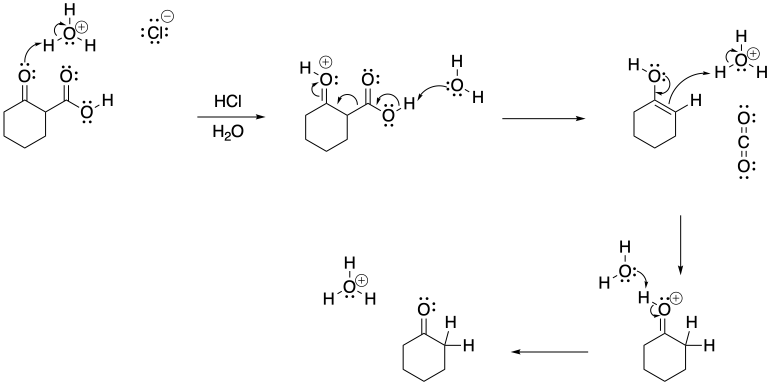
Problem CO12b.15.
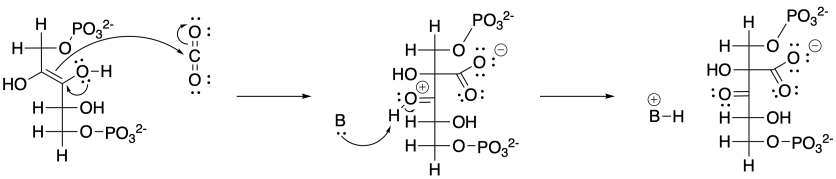
Problem CO12b.16.

Problem CO12c.1.
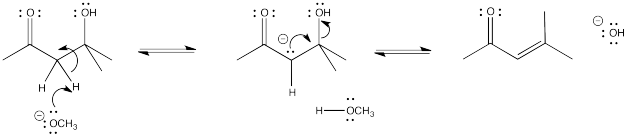
Problem CO12c.2.
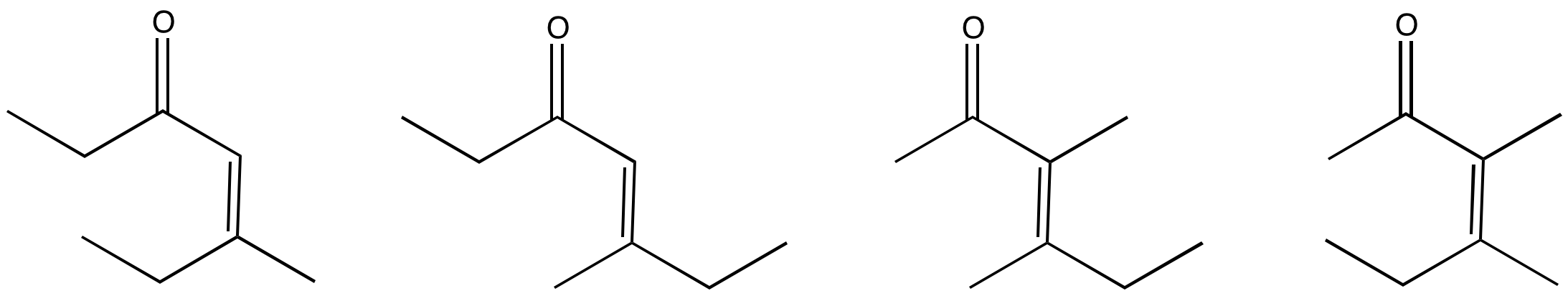
Problem CO12c.3.
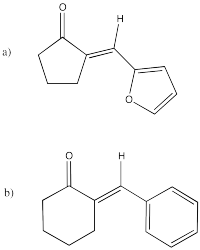
Problem CO12c.4.
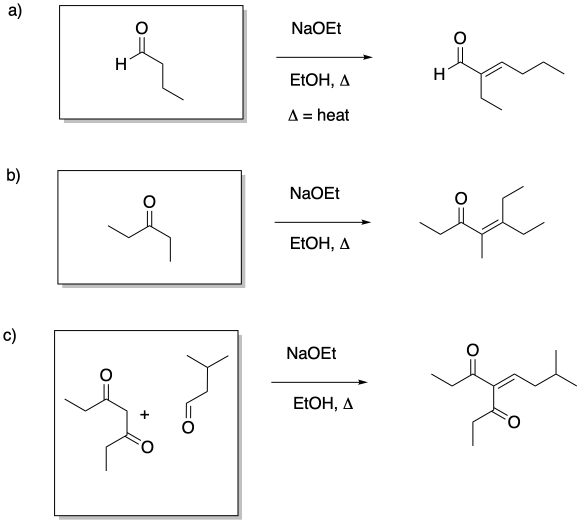
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carbonyl Addition Index
Back to Web Materials on Structure & Reactivity in Chemistry