CO28. Solutions to Selected Problems, Part B
Problem CO13.1.
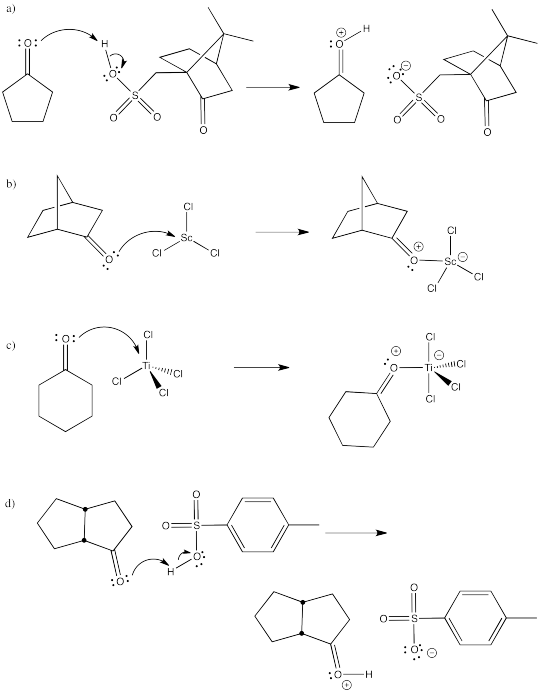
Problem CO13.2.
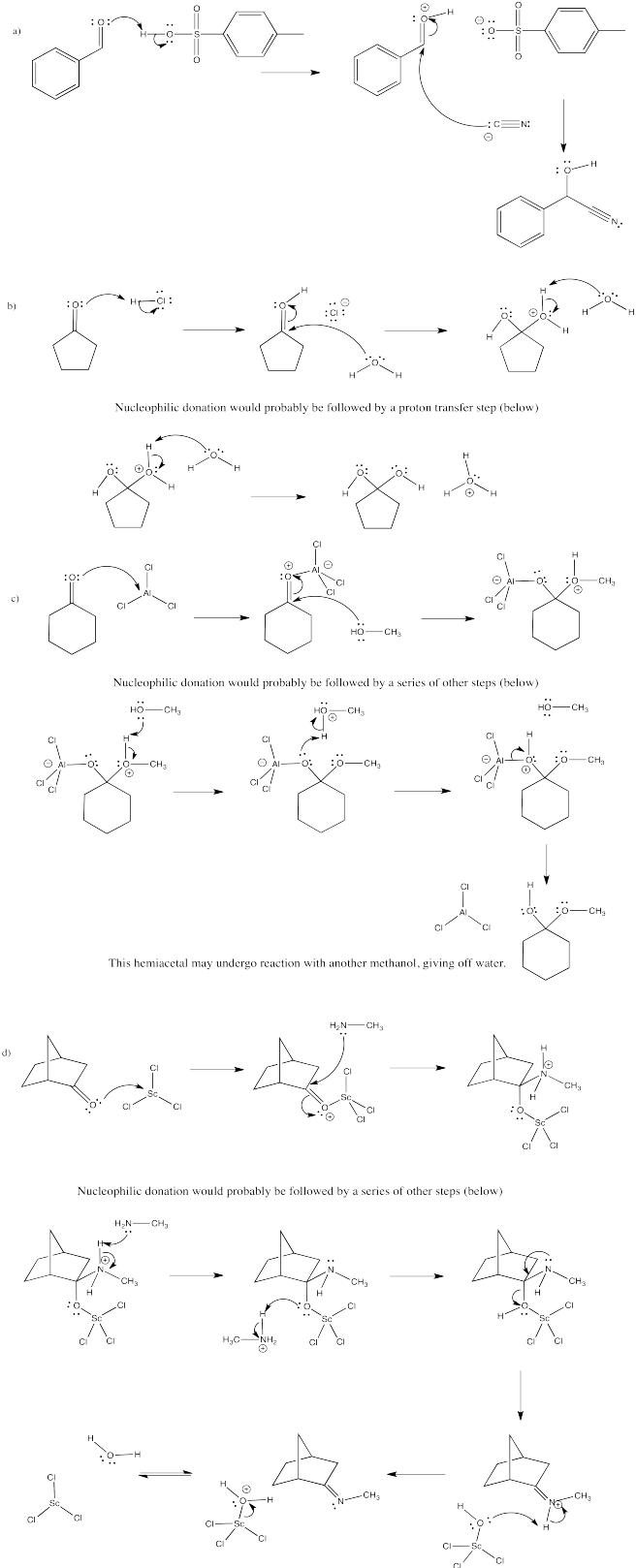
Problem CO13.3.
Part a.
Part b.
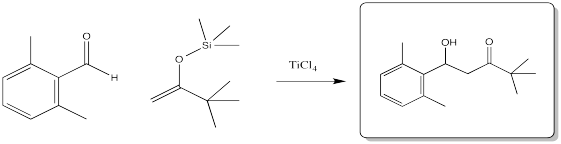
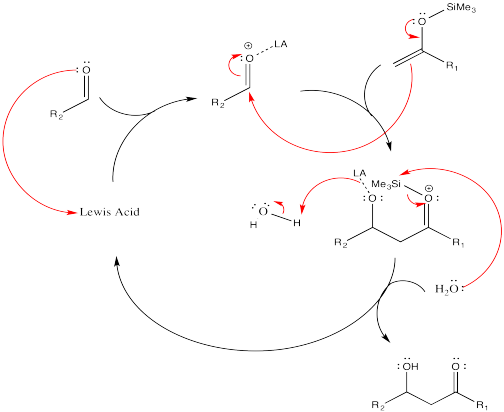
Problem CO13.4.
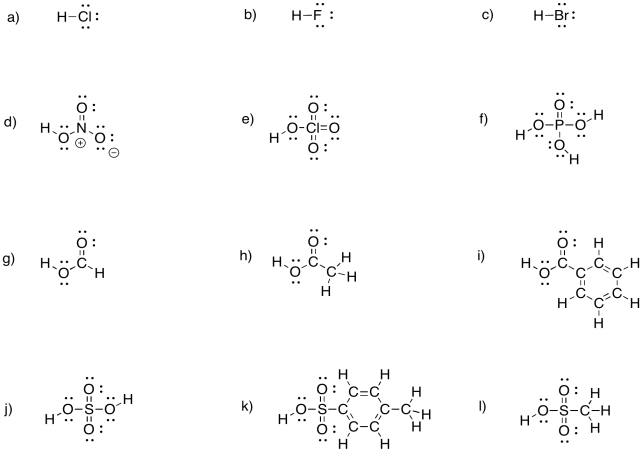
Problem CO14.1.
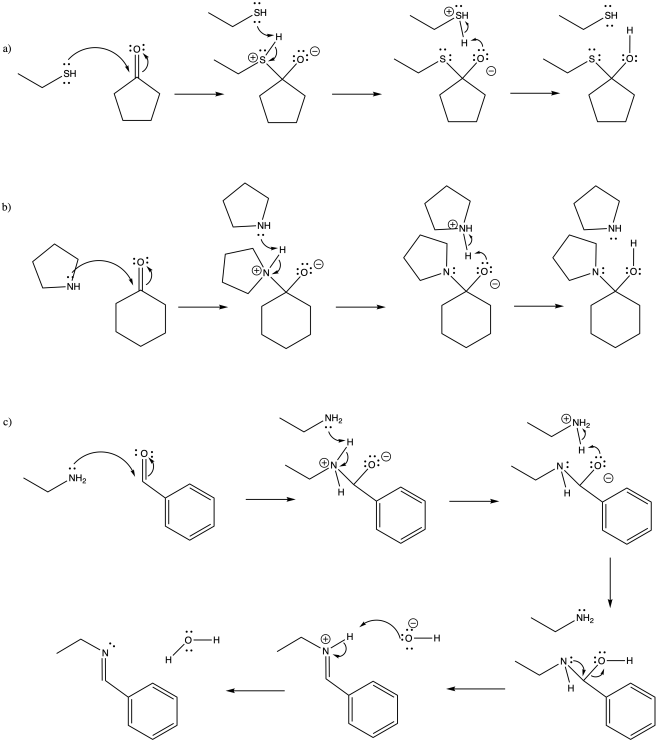
Problem CO14.2.
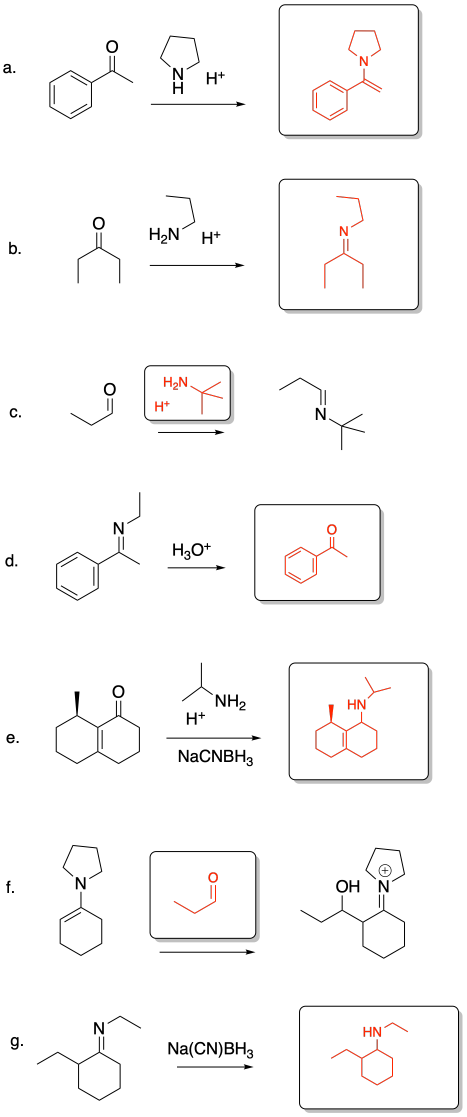
Problem CO14.3.
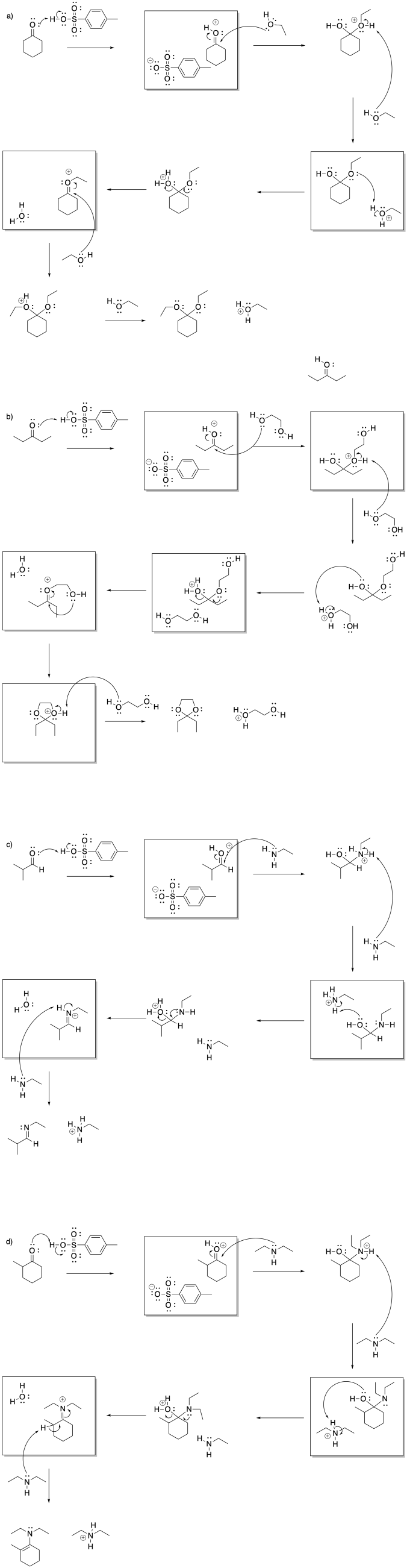
Problem CO14.4.
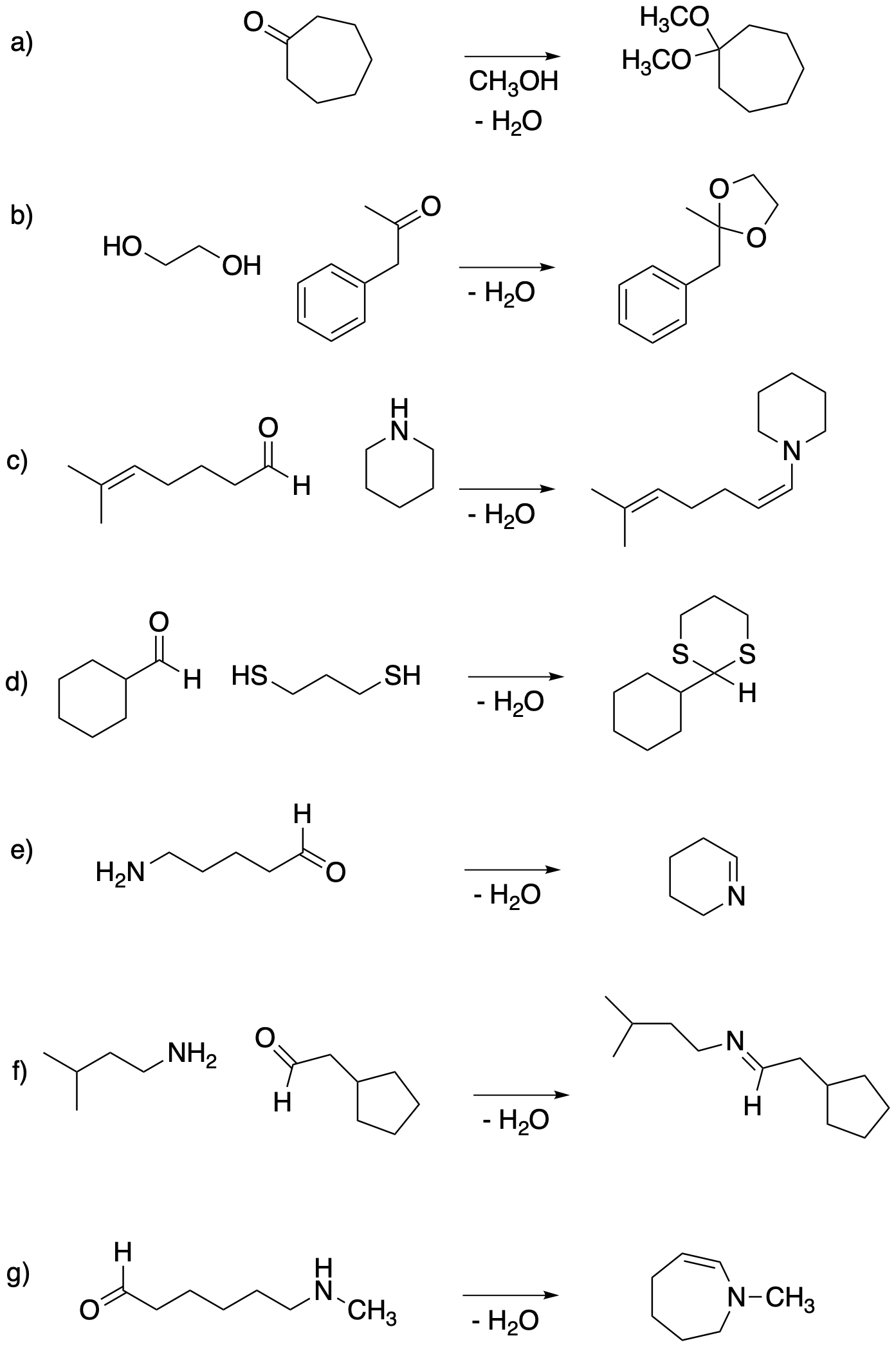
Problem CO15.1.
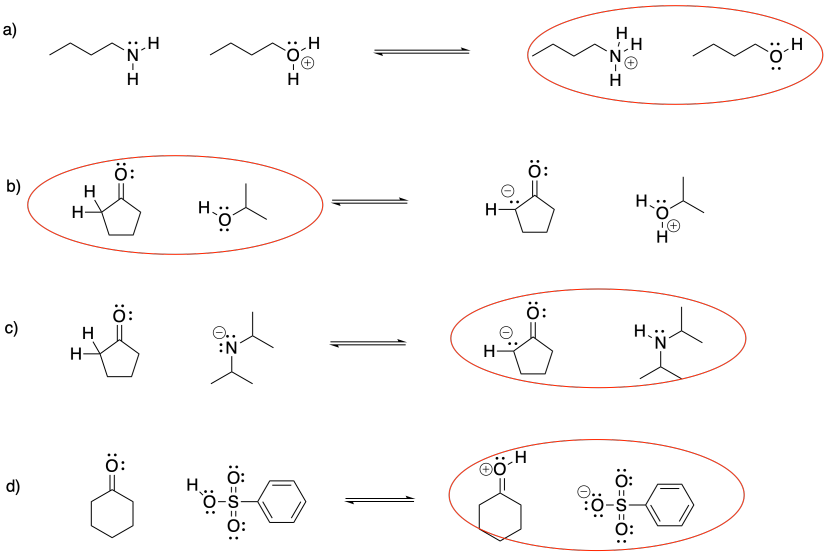
Problem CO15.2.
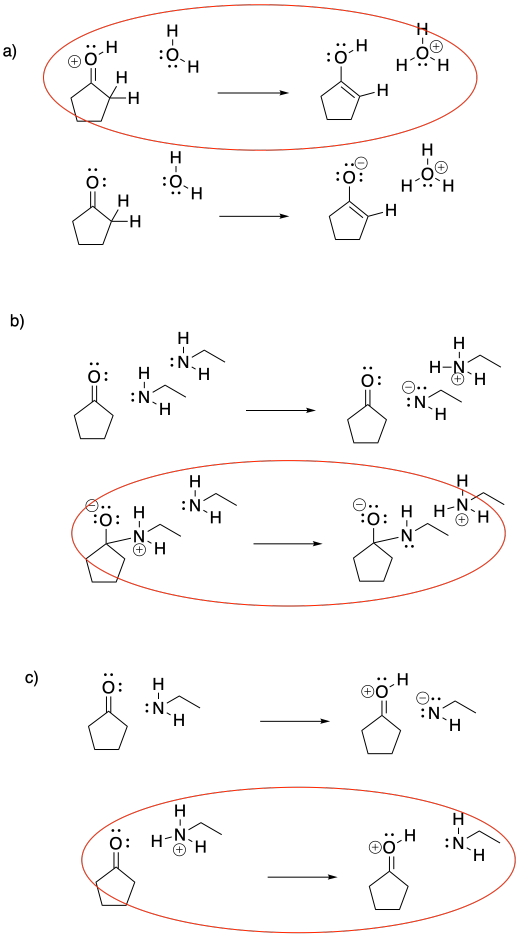
Problem CO16.1.
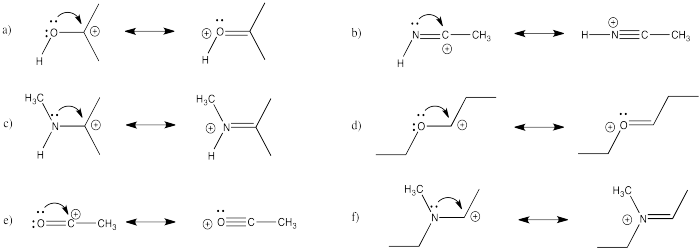
Problem CO16.2.
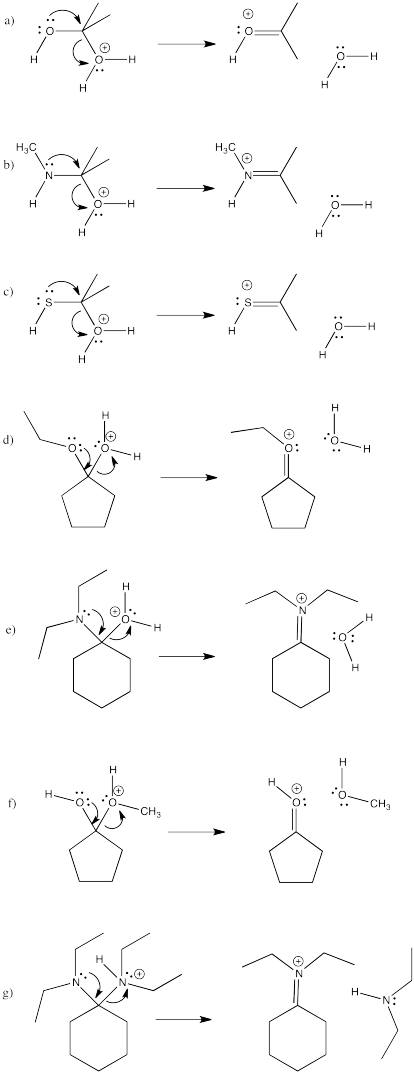
Problem CO16.3.
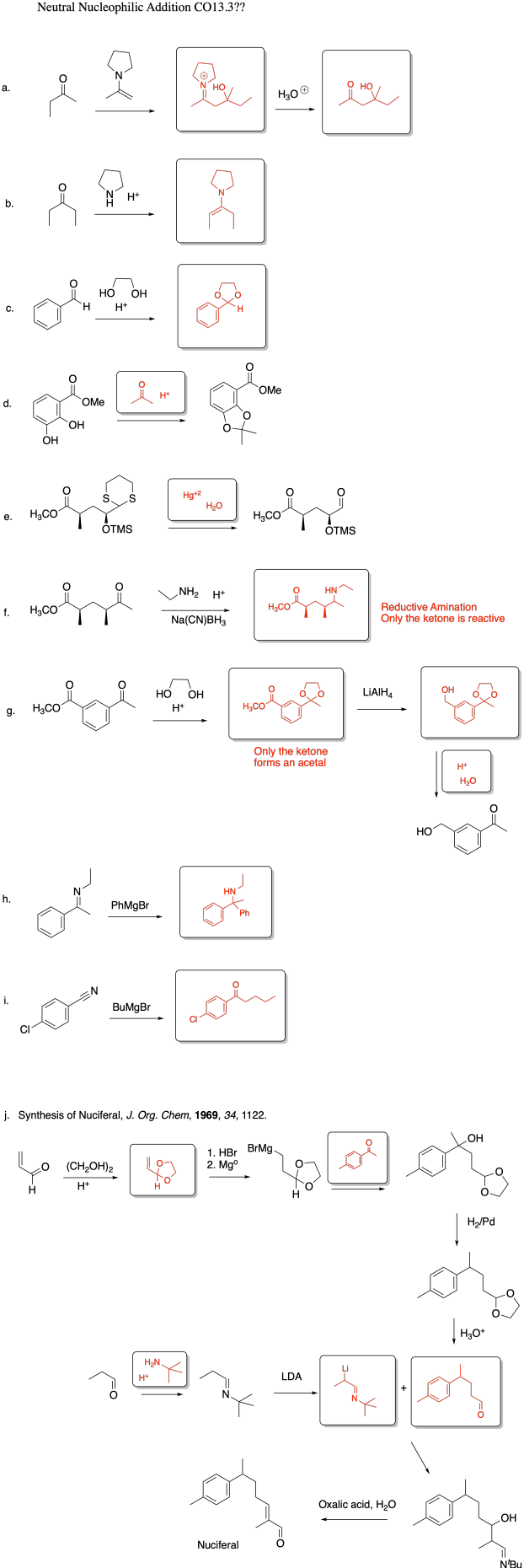
Problem CO18.1.
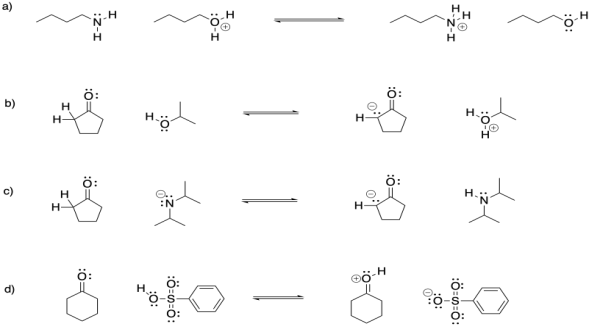
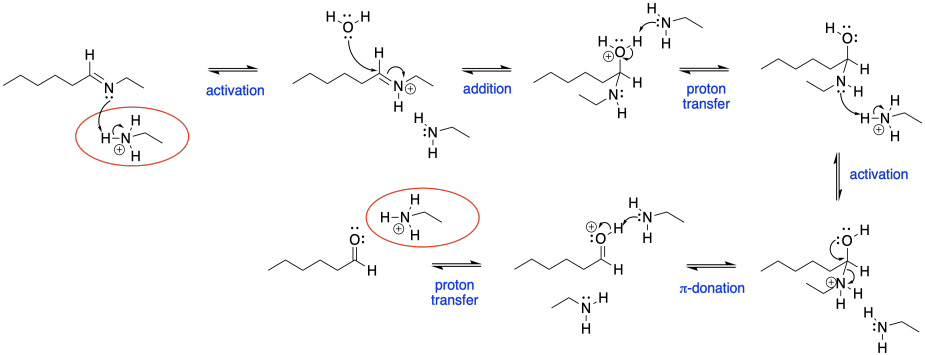
Problem CO18.2.
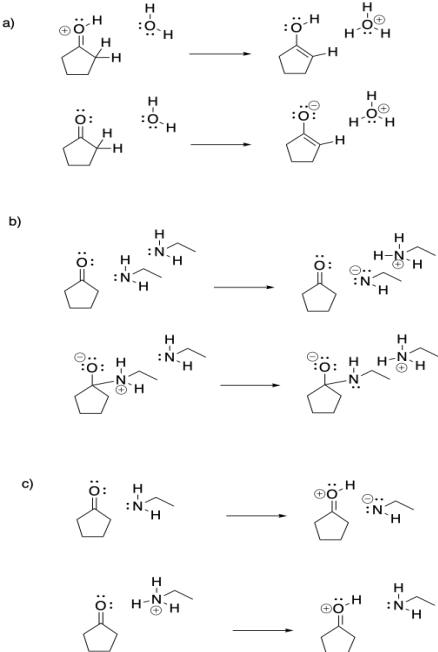
Problem CO16b.1.
Problem CO16b.2.
Problem CO16b.3.
Problem CO16b.4.
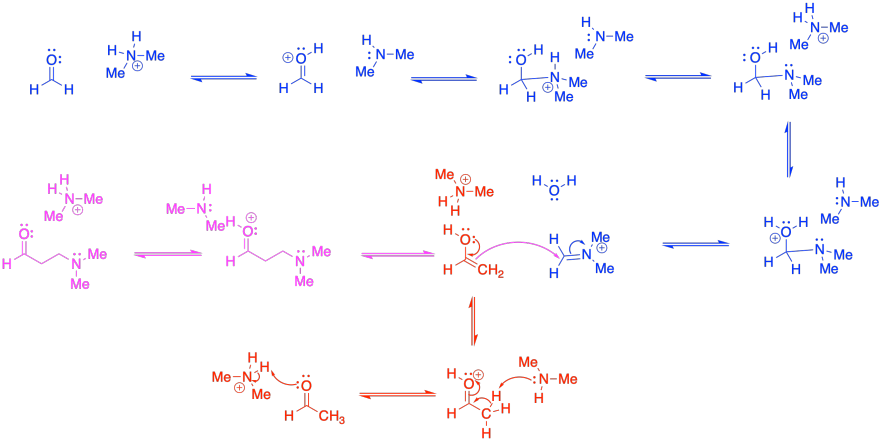
This mechanism requires formation of both an iminium ion (blue) and an enol
(red).
Problem CO18.1.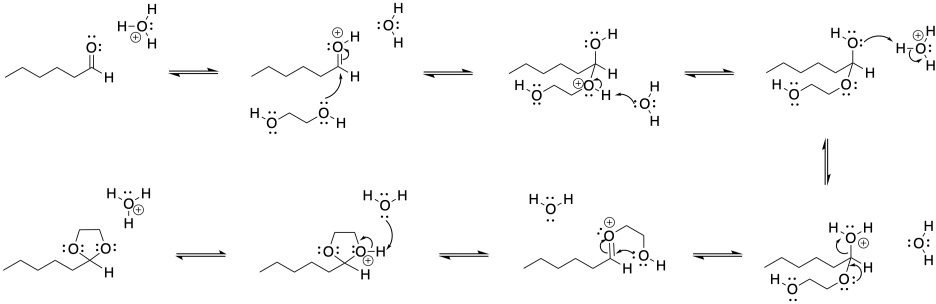
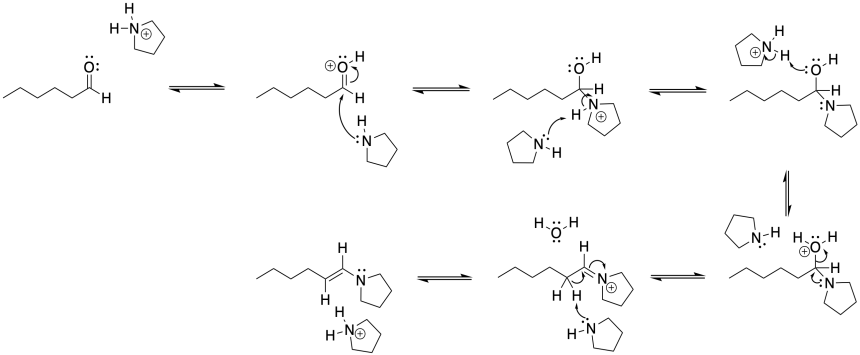
If the OH group on the anomeric position becomes protonated, it is easily pushed out by the oxygen in the ring. A nucleophile would then be attracted to the anomeric carbon.
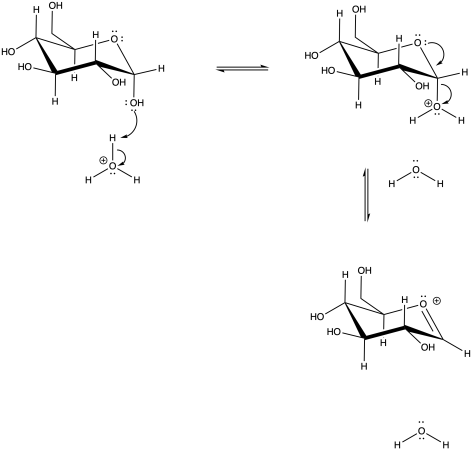
Problem CO18.2.
A nucleophile could approach the electrophilic carbon from either face, leading to both anomers. The result will be a diastereomeric mixture. Sterically, approach from the upper face in the picture, leading to the β-anomer, would be a little more favourable.
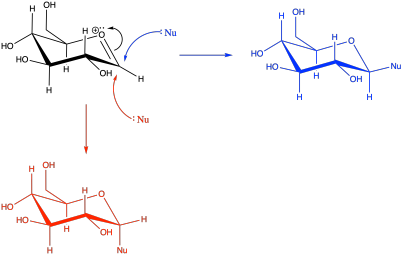
Problem CO18.3.
Phosphorylation occurs at a primary alcohol (RCH2OH). The other hydroxyl sites are secondary alcohols (R2CHOH). There is a significant steric difference between the two sites. Even in the absence of enzyme control (which is surely the case here), the primary site would react more easily than the secondary site, except in special cases such as an anomeric site.
Problem CO18.4.
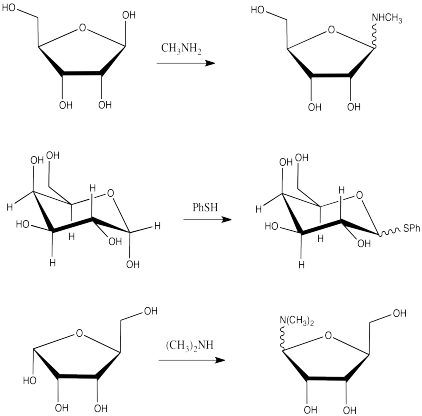
Problem CO18.5.
Problem CO18.6.
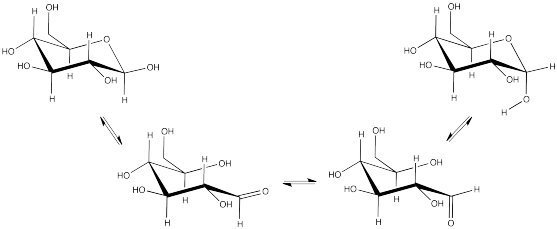
a) α- and β-D-glucopyranose are diastereomers. We cannot a priori predict the specific rotation of pure β-D-glucopyranose based on the specific rotation of α-D-glucopyranose, like we could if they were enantiomers.
b) We do know that, over time, an equilibrium is reached between these two isomers via mutarotation. That means that eventually, the solution would reach a value of 48 degrees, which is based on the contributions of differing amounts of the two diastereomers to the optical rotation.
Problem CO18.7.
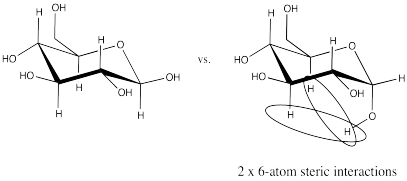
Problem CO18.8.
a) The lone pairs are farther from each other in the alpha anomer than in the beta anomer. That leads to weaker repulsion in the alpha anomer than in the beta anomer, based on Coulomb's Law. Coulomb's Law indicates the energy increase due to repulsion is inversely proportional to distance.
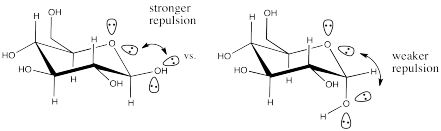
b) In water, lone-pair repulsion will be attenuated because the polar solvent will screen the lone pairs from each other. One lone pair will not experience repulsion from another because of the dipoles of water molecules in between them.
Problem CO19.1.
Problem CO20.6.

Problem CO20.7.
Problem CO21.1.
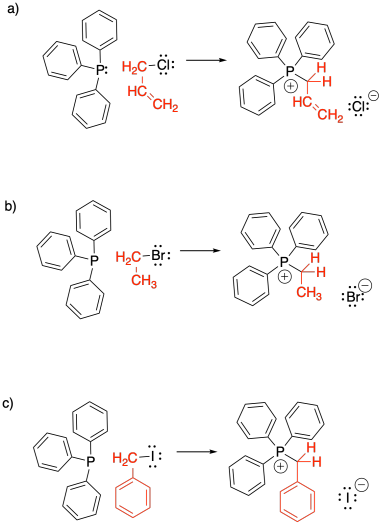
Problem CO21.2.
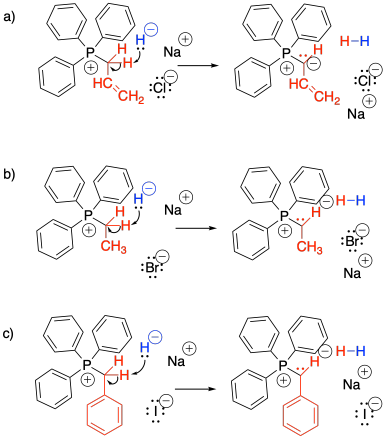
Problem CO21.3.
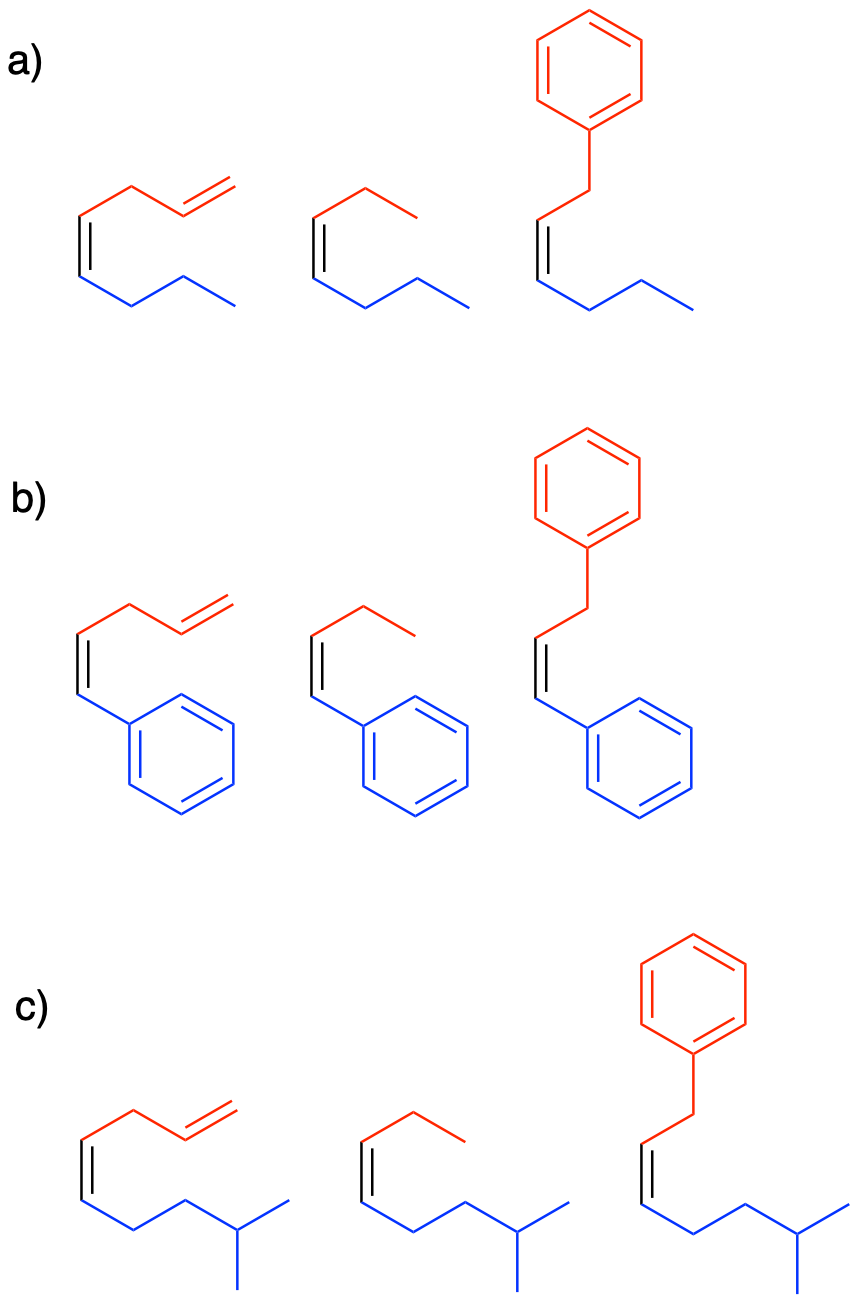
Problem CO21.4.
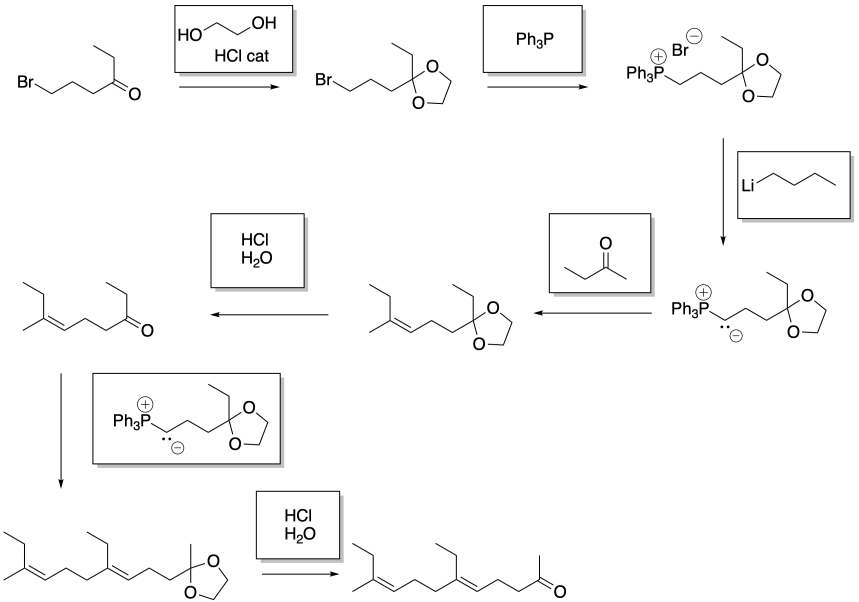
Problem CO21.5.
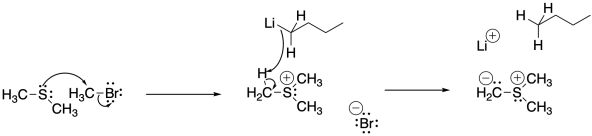
Problem CO21.6.
a)
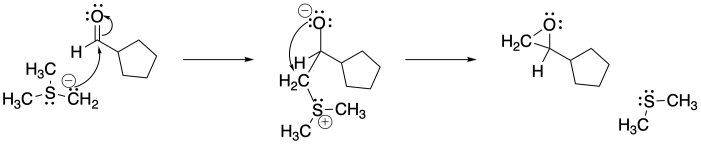
b)
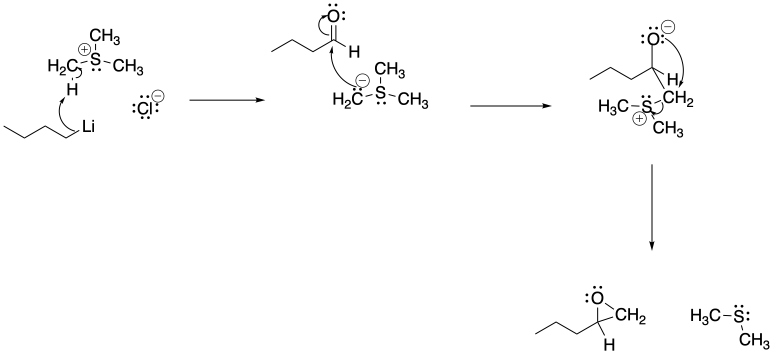
Problem CO21.7.
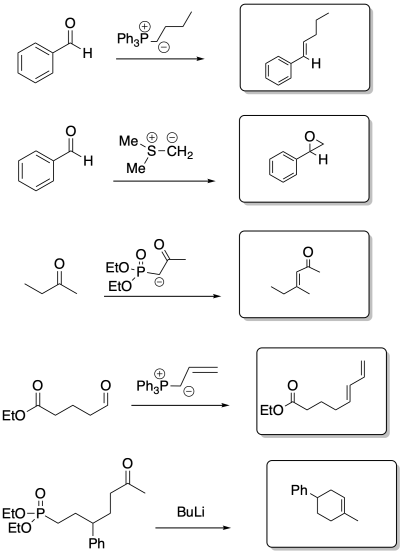
Problem CO21.8.
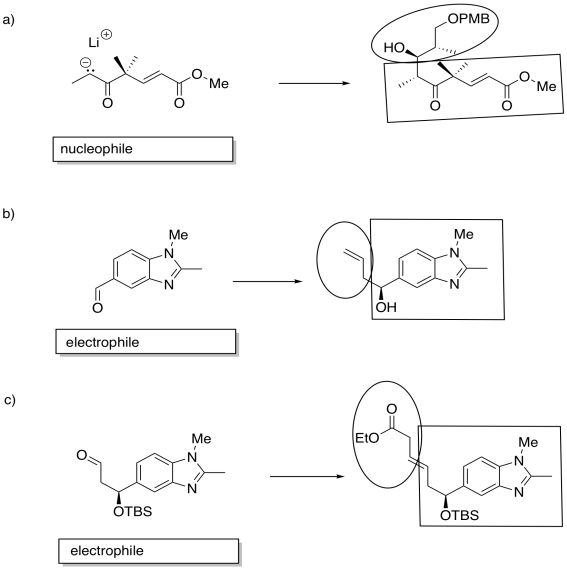
Problem CO21.9.
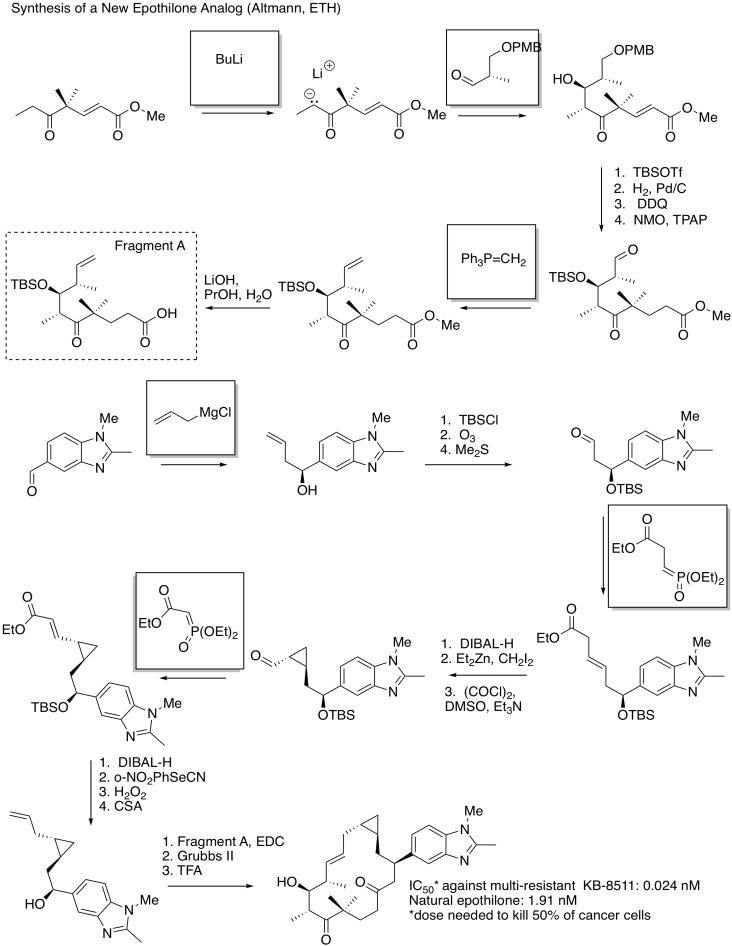
Problem CO21.10.
a)
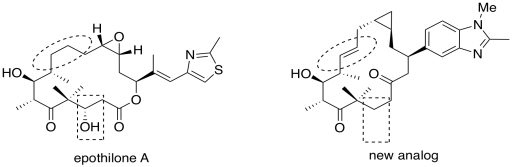
b) The circled part changed from a single bond to a doube bond. The boxed part changed from an alcohol to unadorned hydrocarbon chain.
c) Hydrogen bonding is the most obvious.
d) However, the analog works better without this group; this particular alcohol group is probably not an important part of the pharmacophore. It is probably not needed in order to bind to the target.
e) In epothilone A, as drawn, the dihedral angle appears to be 0 degrees.
f) In the new analog, the dihedral angle is 180 degrees.
g) Based on the superior activity of the analog, the active conformation of the ring is probably more like the one on the right than the one on the left. The circled bond probably adopts a dihedral angle closer to 180 degrees, with the rest of the ring twisting into a shape more like the one shown on the right, in order to bind to the target.
Problem CO22.1.
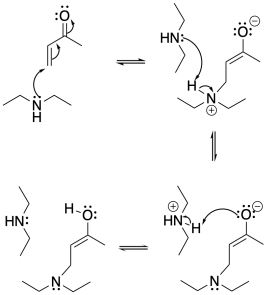
Problem CO22.2.
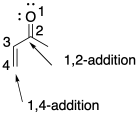
Problem CO22.3.

Problem CO22.4.
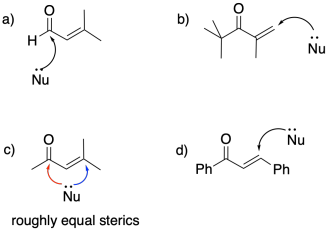
Problem CO22.5.
a) Attachment to copper makes the alkyl a soft ligand. It adds 1,4.
b) Attachment to magnesium makes the alkyl a hard ligand. It adds 1,2.
c) Delocalization makes the enamine a soft ligand. It adds 1,4.
d) Delocalization makes the enolate a soft ligand. It adds 1,4.
Problem CO22.6.
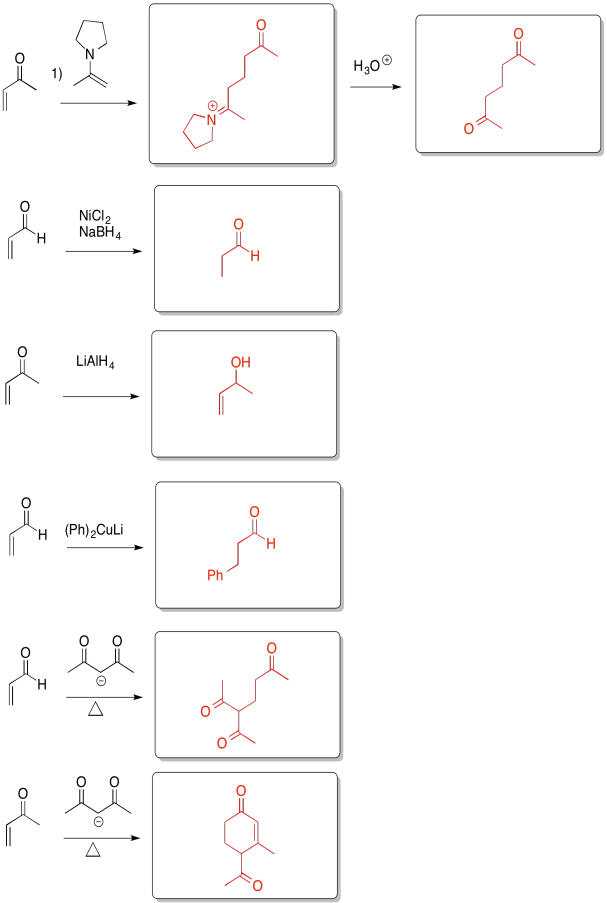
Problem CO22.7.
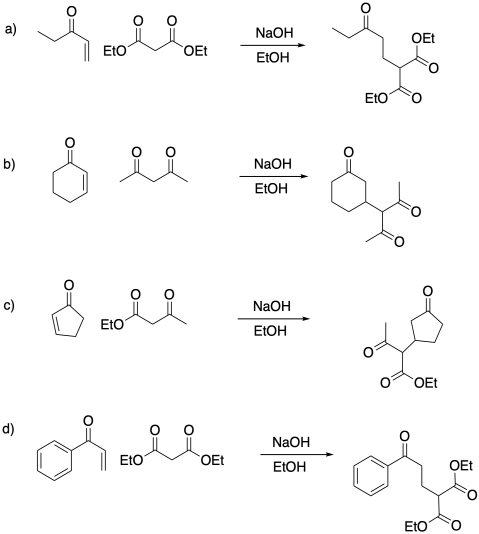
Problem CO22.8.
a)
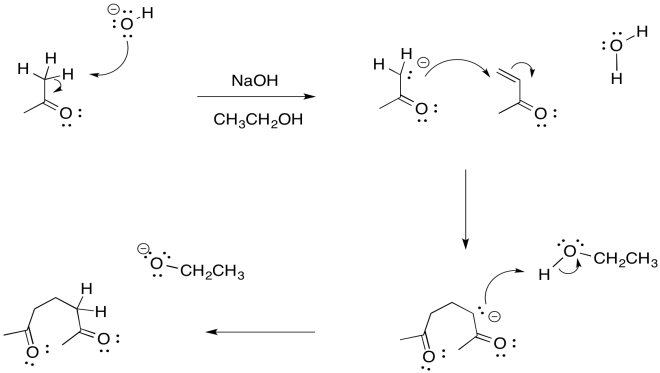
b)
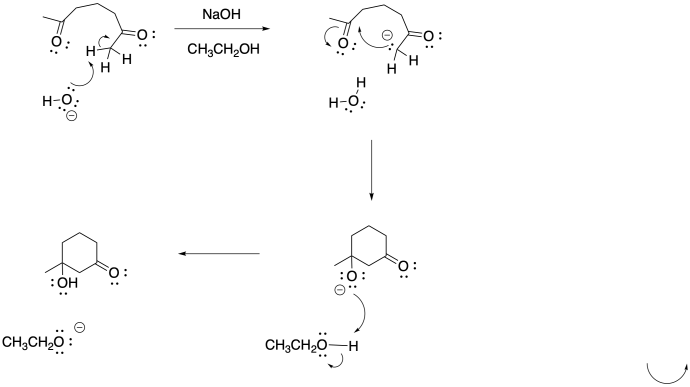
c)
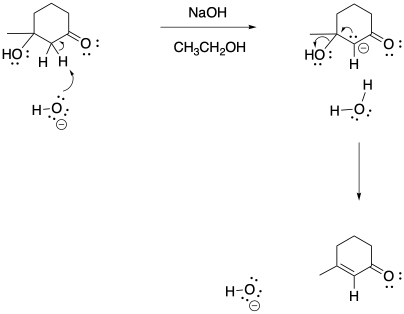
Problem CO22.9.
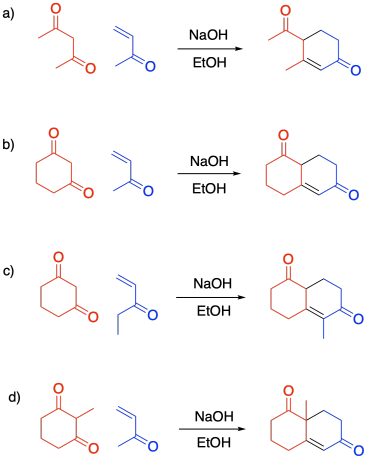
Problem CO22.10.
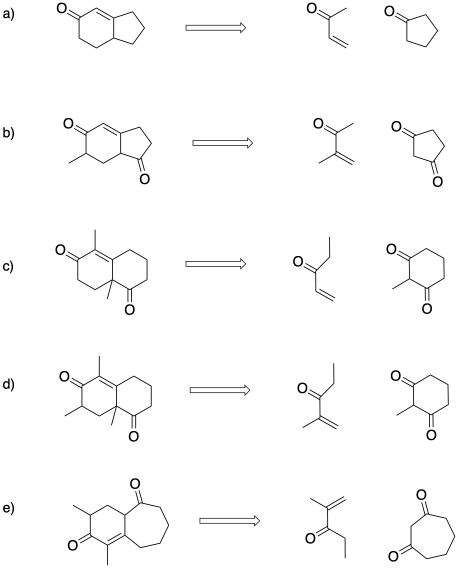

Problem CO23.1.
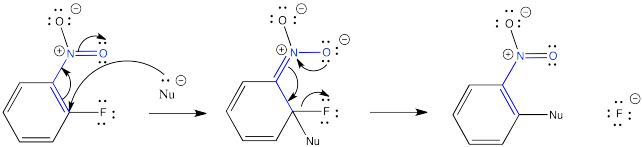
Problem CO24.1.
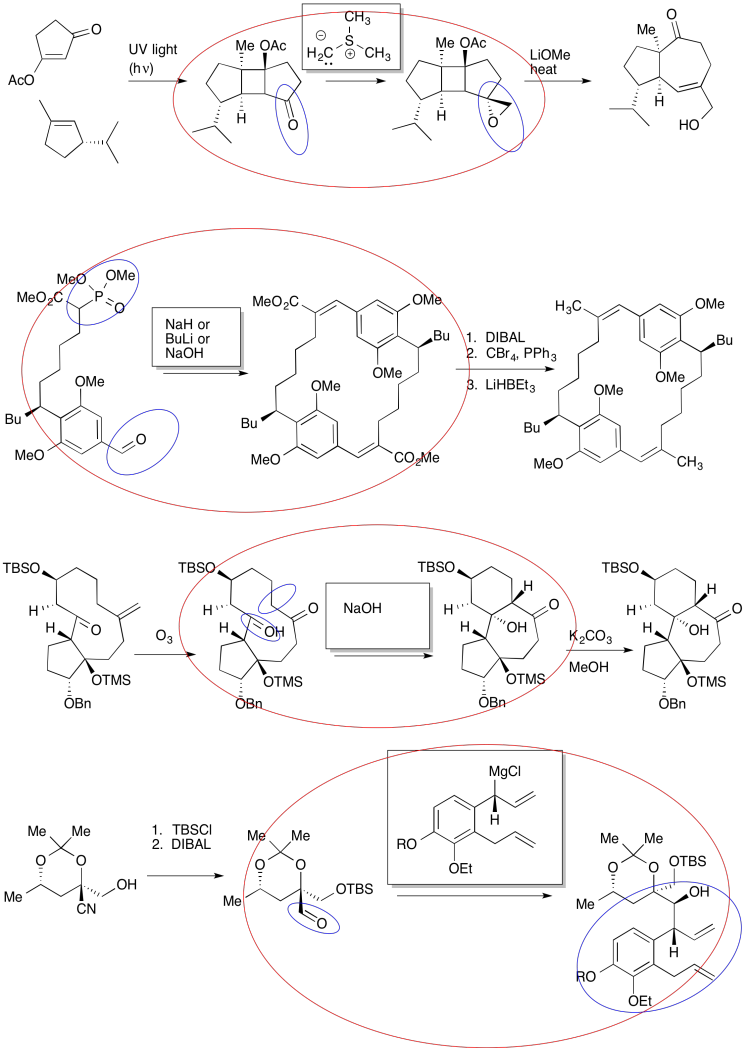
Problem CO24.2.
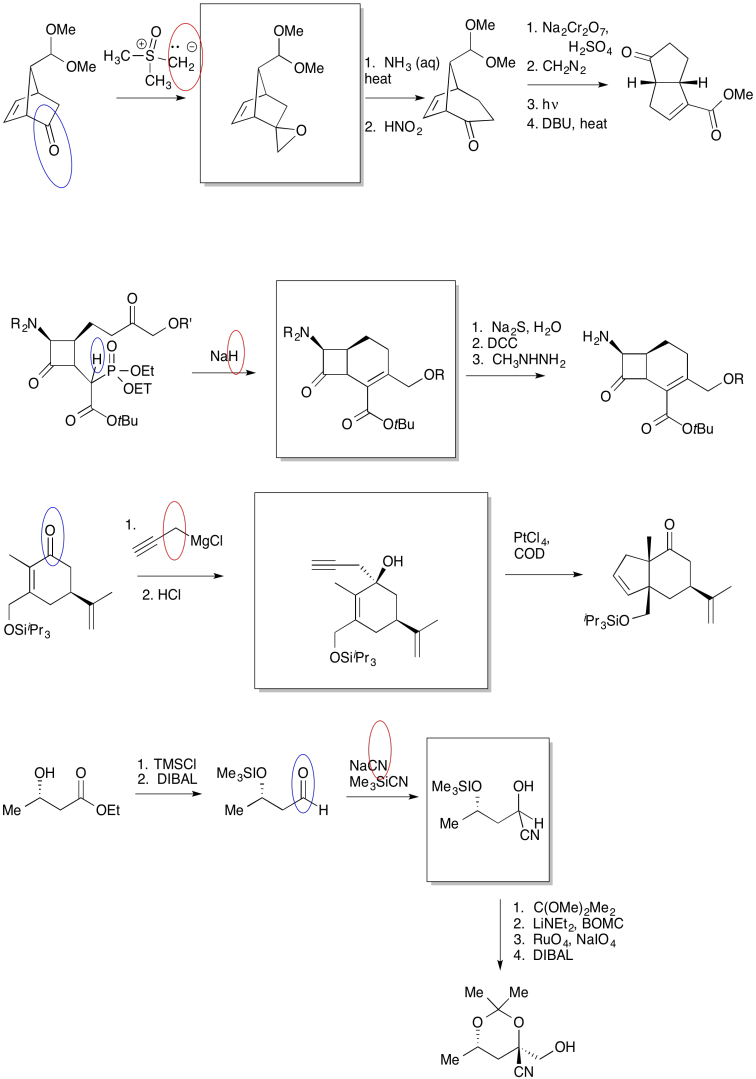
Problem CO24.3.
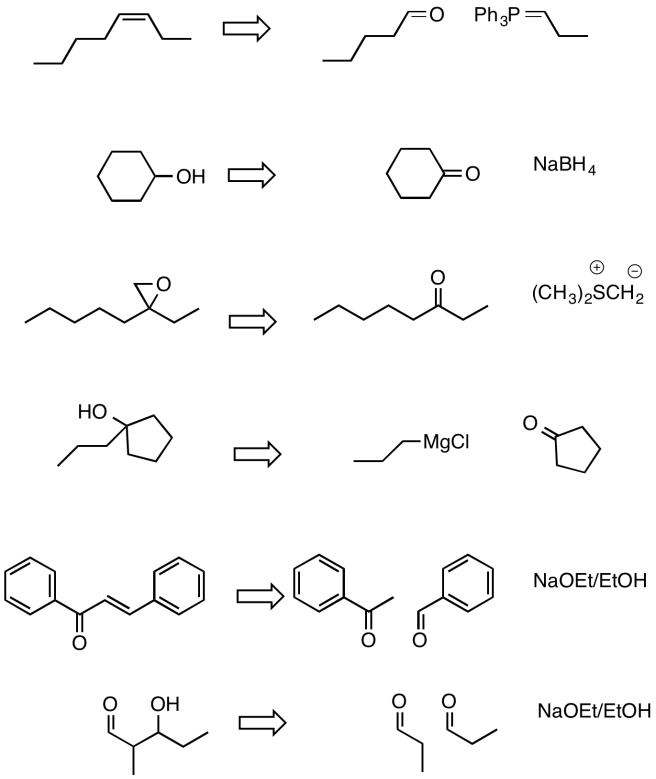
Problem CO24.4.
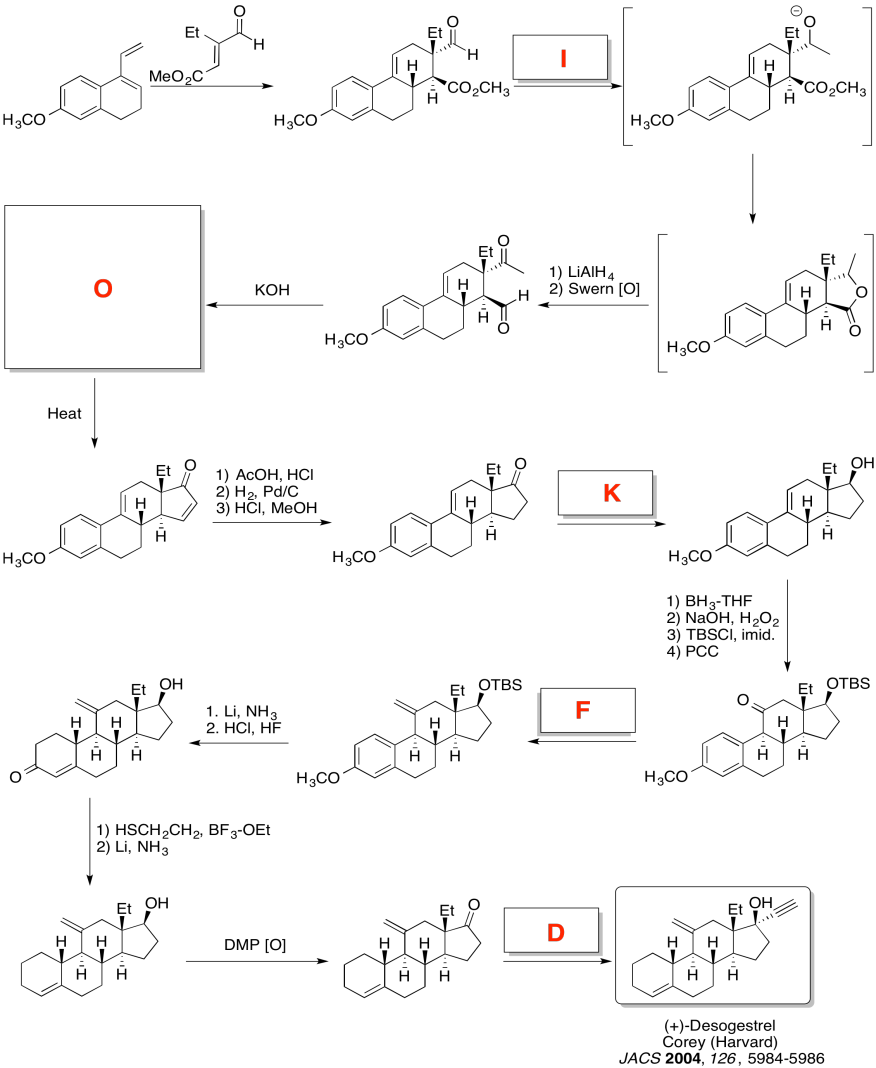
Problem CO24.5.
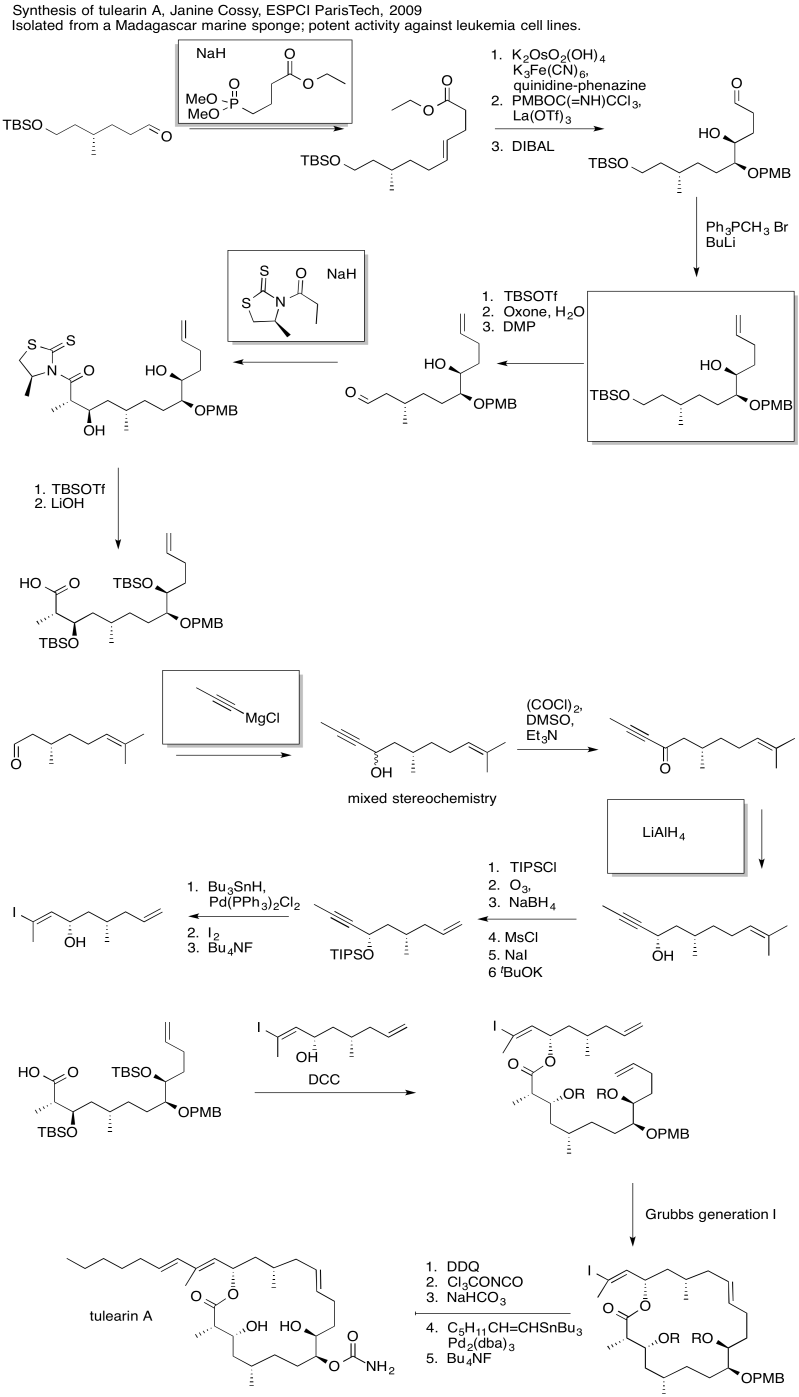
Problem CO24.6.
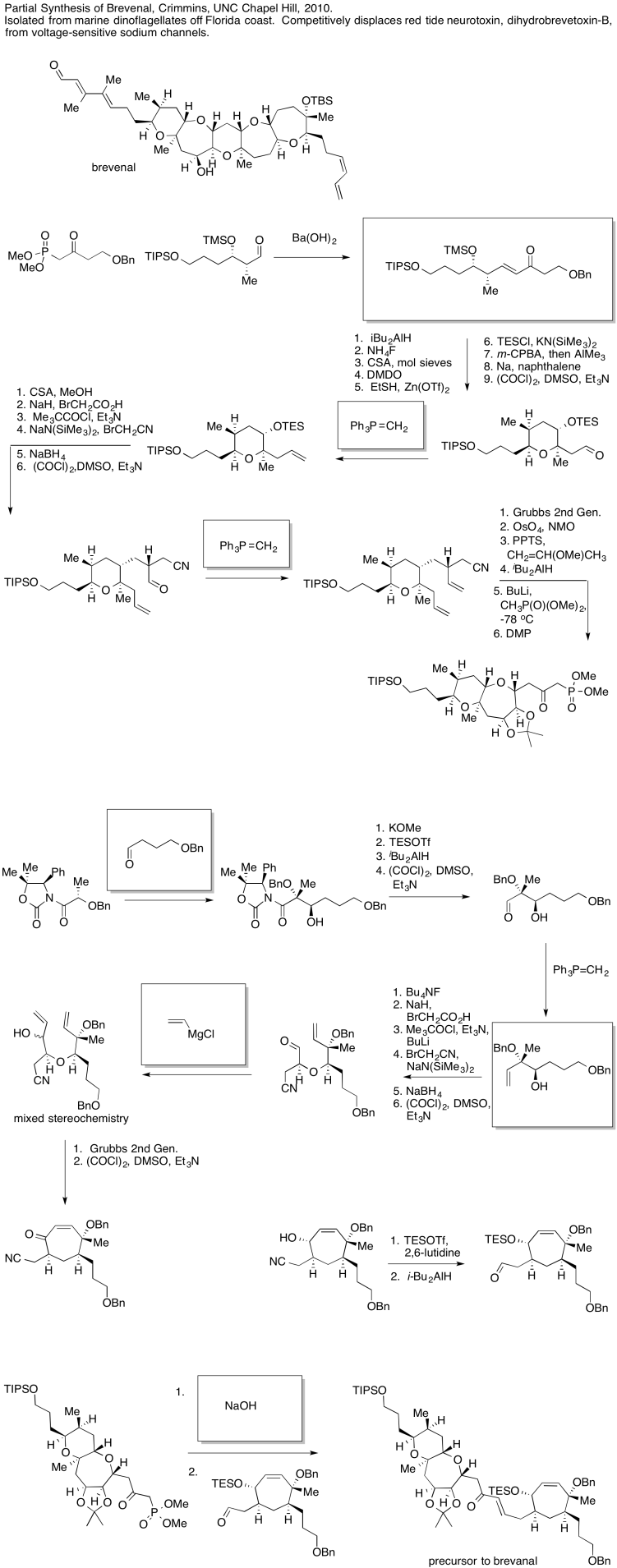
Problem CO26.1.
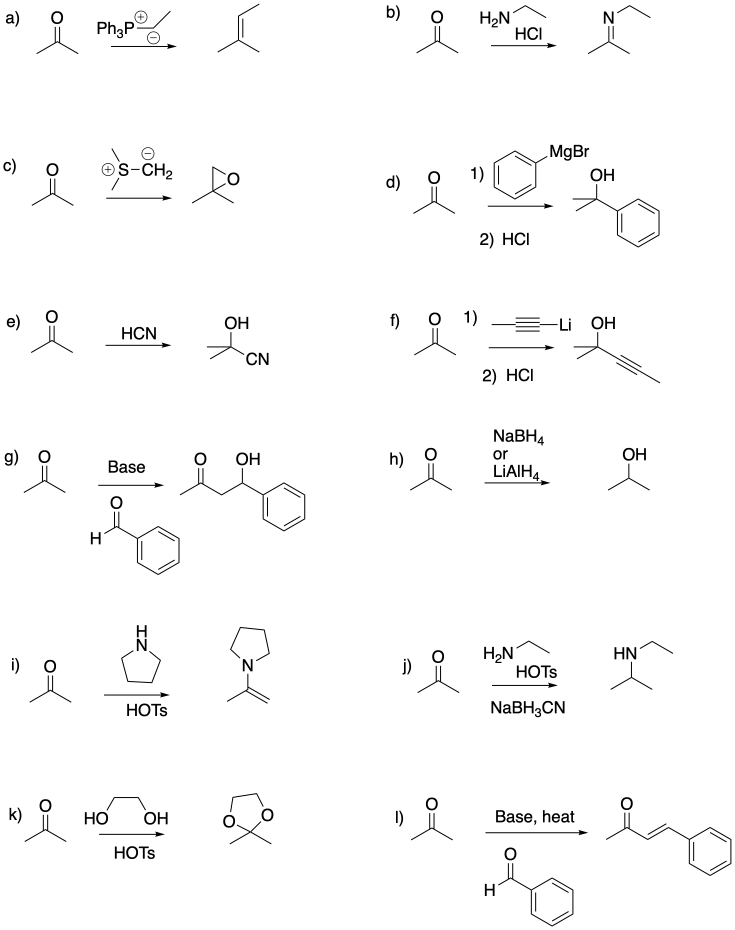
Problem CO26.2.
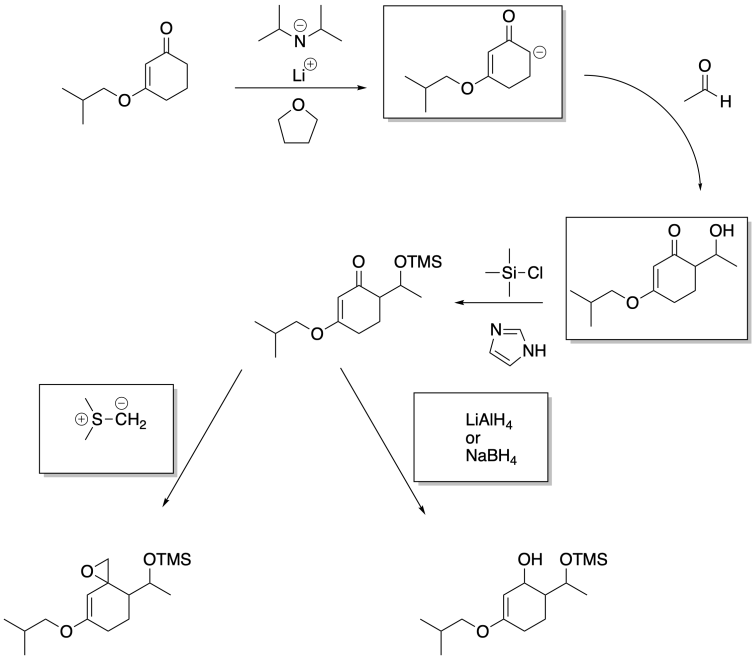
Problem CO26.3.
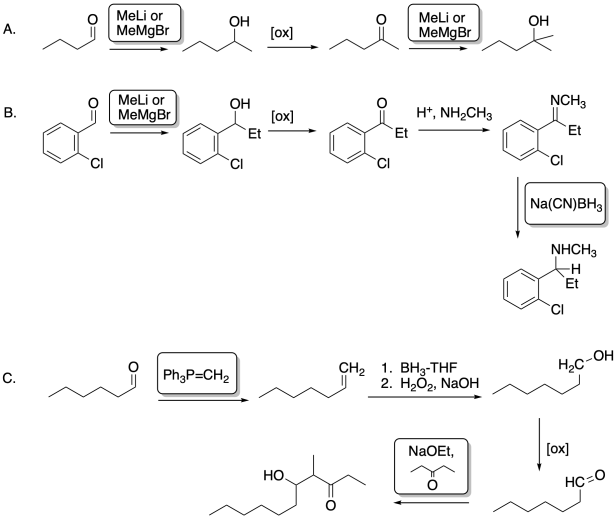
Problem CO26.4.
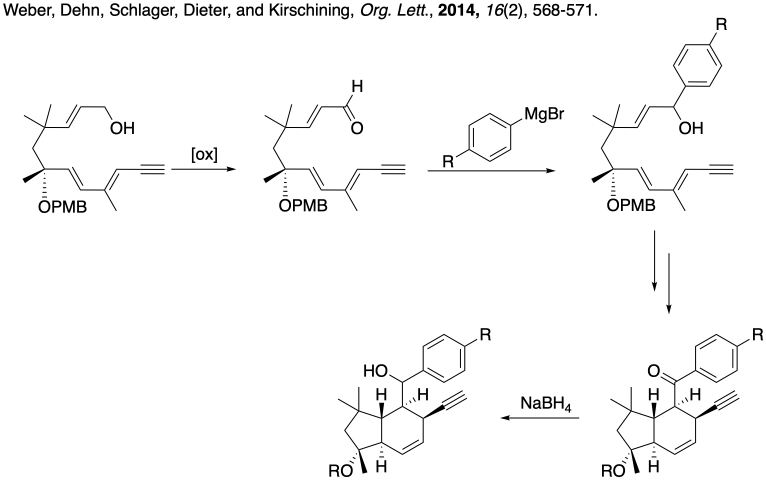
Problem CO26.5.
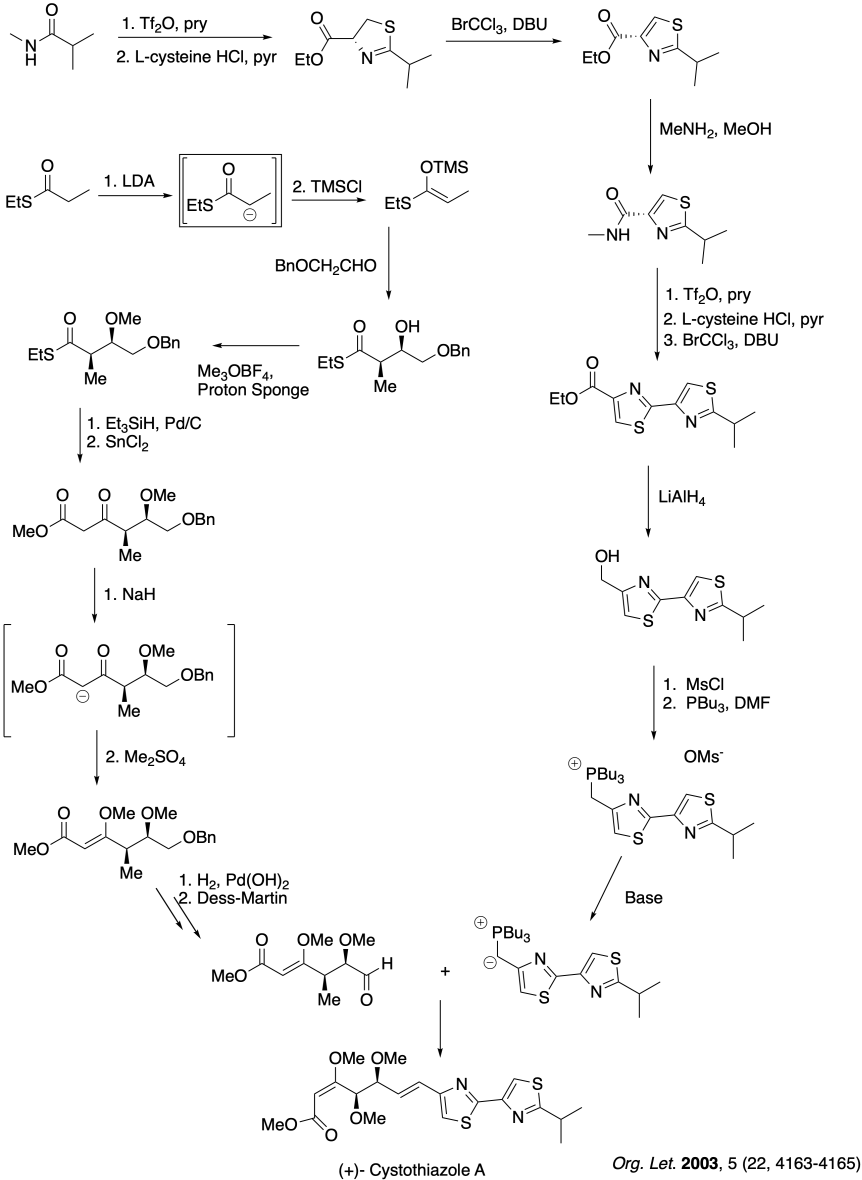
Problem CO26.6.
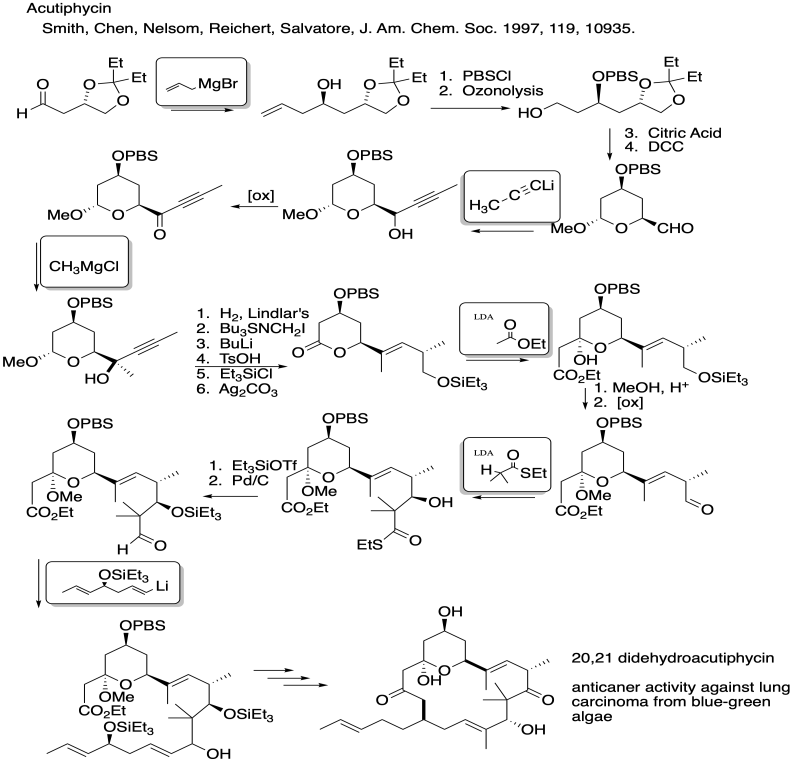
Problem CO26.7.
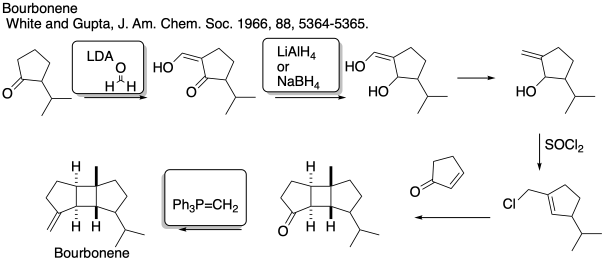
Problem CO26.8.
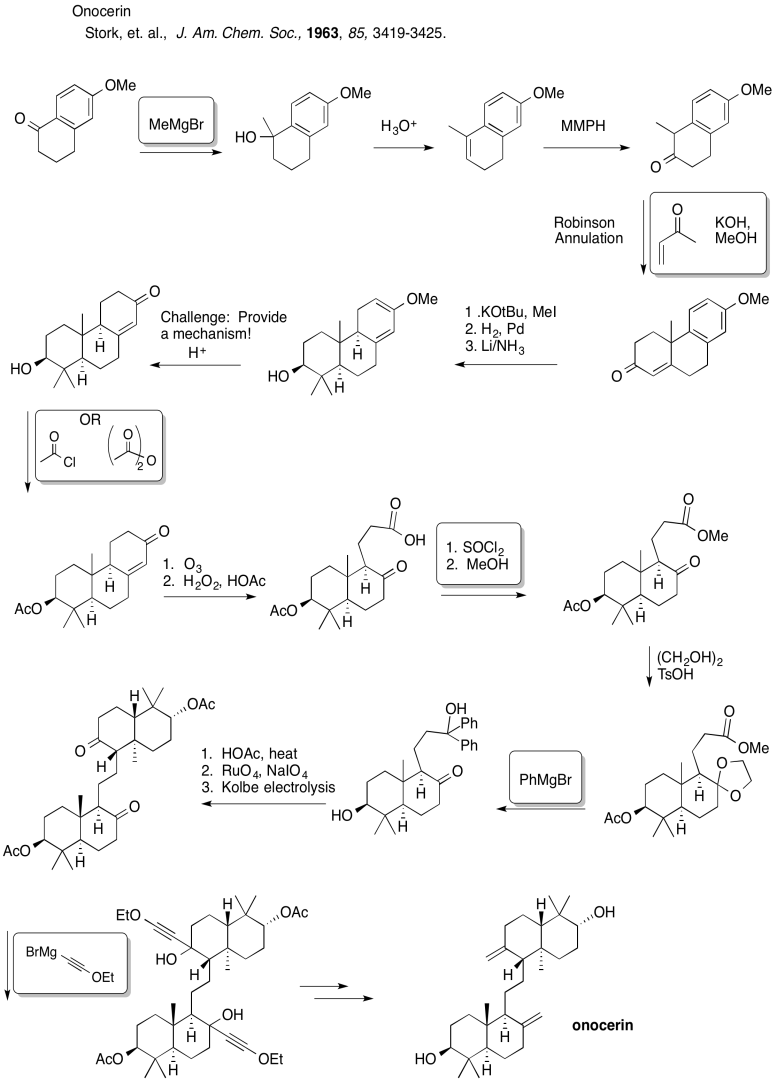
Problem CO26.9.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carbonyl Addition Index
Back to Web Materials on Structure & Reactivity in Chemistry