Reactivity in Chemistry
Substitution at Carboxyloids
CX1. Introduction to Carboxyloids
Aldehydes and ketones are carbonyl compounds in which the carbonyl carbon is connected only to carbons atoms (in the case of a ketone) or to one carbon and one hydrogen atom (in the case of an aldehyde).
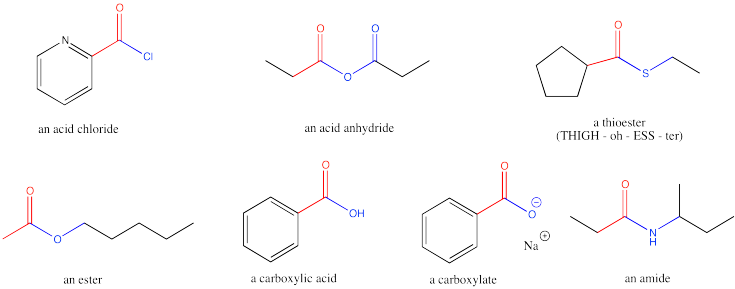
Carboxyloids, or carboxylic acid derivatives, are carbonyl compounds in which the carbonyl carbon is attached to one carbon and one heteroatom. Most commonly, the heteroatom is an oxygen, nitrogen, sulfur or chlorine.

Figure CX1.1. The carboxyloids or carboxylic acid derivatives.
Carboxylic acid derivatives were historically thought of as being made from carboxylic acids; hence the name. That name is a mouthful; we will use the alternative "carboxyloids", a term coined by the 20th century physical organic chemist, Christopher Ingold.
The reactivity of carboxyloids is typically different from aldehydes and ketones. Because of this difference it is useful to study these compounds separately from the simple carbonyls.
Problem CX1.1.
What are some things that the heteroatoms involved in carboxyloids have in common?
Like simple carbonyls, carboxyloids react with nucleophiles. Just like simple carbonyls, the LUMO of a carboxyloid is usually the C=O pi antibonding orbital (the π*). Populating this orbital with a pair of electrons from the nucleophile results in breaking the C=O pi bond, leaving only a C-O sigma bond.
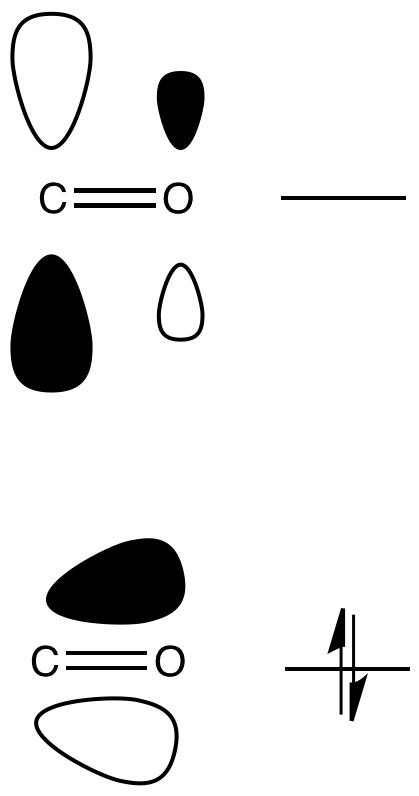
Figure CX1.2. The LUMO of a carboxyloid.
Remember that this addition to the C=O bond is often reversible. In the case of carboxyloids, however, re-forming the pi bond can go through two pathways. In one pathway, the nucleophile can be displaced, returning to starting materials. In another pathway, the group attached to the carbonyl can be displaced instead. In that pathway, a new product results. This step in the pathway is sometimes called the elimination step, incontrast to the addition step.
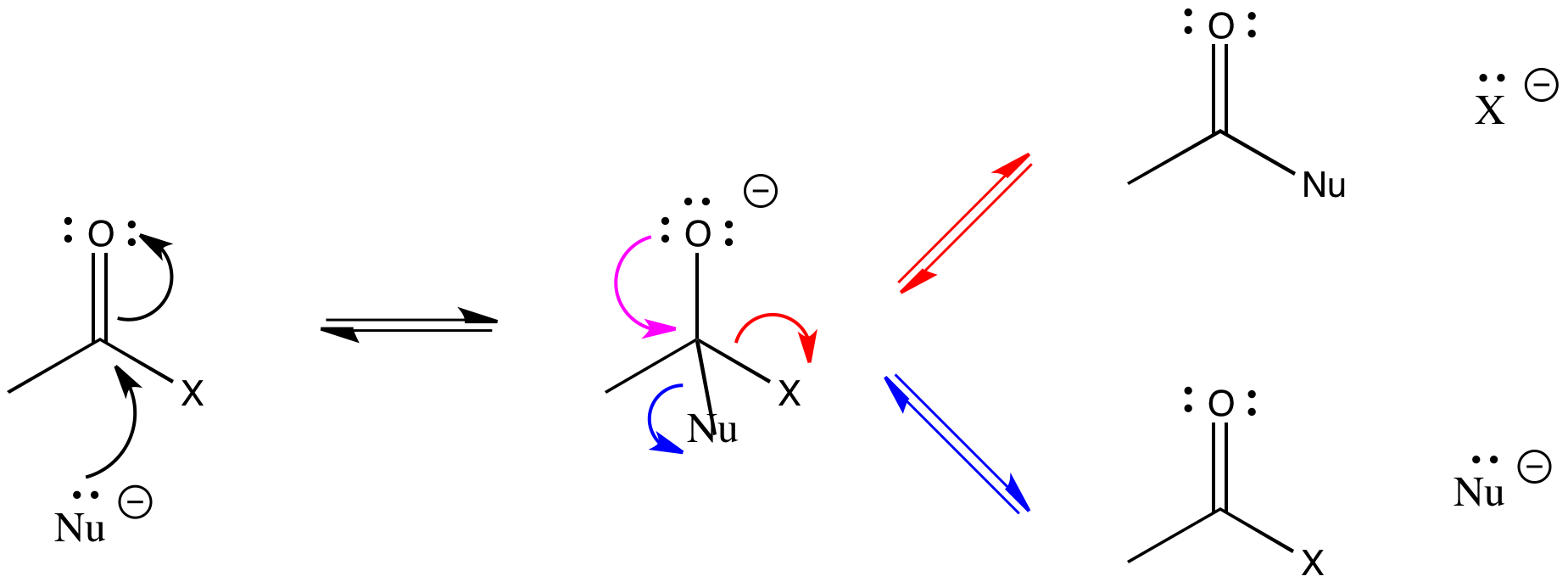
Figure CX1.3. Possible elimination pathways.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carboxyl Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry