Reactivity in Chemistry
Substitution at Carboxyloids
CX12. Solutions For Selected Problems
Problem CX1.1.
These heteroatoms are all found in the upper right-hand corner of the periodic table. They are all pretty electronegative and they all have lone pairs.
Problem CX2.1.
a) The electronegativity of the heteroatom attached to the carbonyl group in a carboxyloid is one factor that allows it to leave and form its own stable anion.
b) Although carbon and hydrogen are more electronegative than many of the elements in the periodic table, they are not stable enough as anions to form easily on their own.
Problem CX2.2.
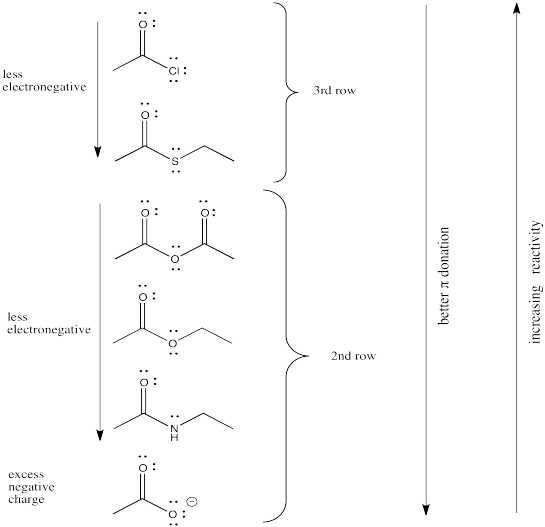
The reactivity order of the carboxyloids shown are, from most reactive to least:
RC(O)Cl > RC(O)SR' > RC(O)OR' ~ RC(O)OH > RC(O)NR'R"
That is, acid chloride > thioester > ester ~ carboxylic acid > amide.
The order is determined by ability of the heteroatom to pi-donate to the carbonyl.
Problem CX2.3.

Problem CX3.1.
We might expect carboxyloids with the most electronegative elements attached to the carbonyl to be the most reactive and least stable towards substitution (in other words, carboxyloids with the most electronegative heteroatoms would become substituted the most easily).
In that case, we would predict that the carboxyloids with the most electronegative substituent (oxygen) would be the most reactive. There are a number of different kinds and we will think about how they relate to each other shortly.
After the oxygen derivatives we would predict either the nitrogen derivatives or the chloride, depending on what electronegativity scale we happen to use (remember, electronegativity is not an experimentally pure property, but the result of a calculation that can be performed in different ways). The sulfur derivative would be least reactive.
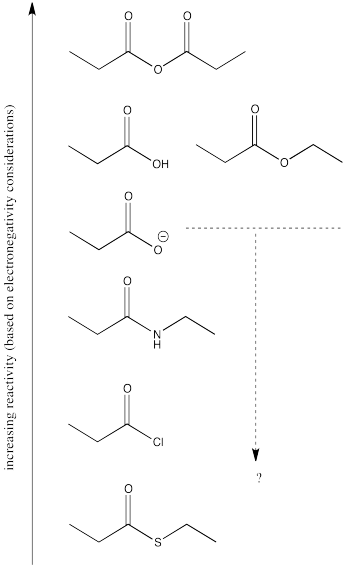
There are still several different oxygen derivatives to compare: carboxylic acids (OH), carboxylates (O-), esters (OR, in which R is an alkyl or carbon chain) and acid anhydrides (OC=O). The easiest to differentiate is the carboxylate, because of its negative charge. It must be less attractive to a nucleophile than the other oxygen derivatives, because it would offer more repulsion to an incoming lone pair.
However, we can't really predict whether it would be any less reactive than the nitrogen, chlorine or sulfur analogues, because who knows whether the charge or the nature of the atom matters more?
As it happens, the charge probably matters more. We learn that simply by looking at the experimental trend and seeing that the carboxylate is the least reactive of all the carboxyloids.
Turning to the other three oxygen derivatives, it would be difficult to differentiate between the effect of a remote hydrogen atom versus an alkyl chain in the ester versus the carboxylic acid, so we'll say those two are about the same. On the other hand, the additional electron-withdrawing carbonyl group in the acid anhydride probably has a profound effect, so we would expect that compound to attract nucleophiles more strongly.
Of course, the series we have produced above is not the "right answer". It does not match the experimentally observed series of carboxyloid reactivities. Nevertheless, it is very useful in terms of building an understanding of carboxyloids. It tells us that electronegativity may play a role here, but that it can't be the only factor.
Some other factor is putting some of the derivatives out of order. In particular, the acid chloride (C=OCl) and the thioester (C=OSR) do not fit.
Electronegativity is an abvious factor that could influence an atom's ability to π-donate, but we just looked at that factor in the previous section, so let's look at another atomic property instead. Of course, different atoms have different sizes. In particular, if we look at the atoms involved in carboxyloid substituents, we can divide them into 2nd row atoms and 3rd row atoms.
It's actually well-documented that the degree of overlap between two orbitals influences how well they bond together. Since carbon is in the second row, it is about the same size as, and overlaps pretty well with, other second row atoms. Third row atoms are a little too big, on the other hand.
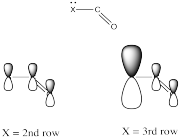
That factor breaks the carboxyloids into two different groups. Assuming π-donation is a major factor, sulfur and chlorine may be placed above the others in tems of reactivity. They cannot donate as well as oxygen or nitrogen can.
From there, differences among the atoms from the same row may be sorted out based on electronegativity differences.
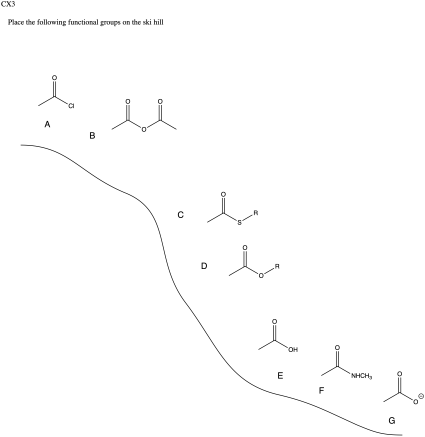
Problem CX3.3.
Amide bonds are among the most stable carboxyloids possible. That stability makes them well-suited to form useful structures that will not decompose easily. Remember, any change that occurs in matter occurs through chemical reactions, including the formation and decomposition of biomaterials. Shutting down a potential chemical reaction means a material will be more durable.
Problem CX3.4.
Problem CX3.5.
One possibility is the presence of an additional electronegative substituent. In the oxalyl chloride, the presence of an additional carbonyl next to the electrophilic acid chloride group would make each carbonyl even more electrophilic. In the thionyl chloride, the presence of two chlorines, instead of just one, could make this compound much less stable and more electrophilic.
Problem CX4.1.
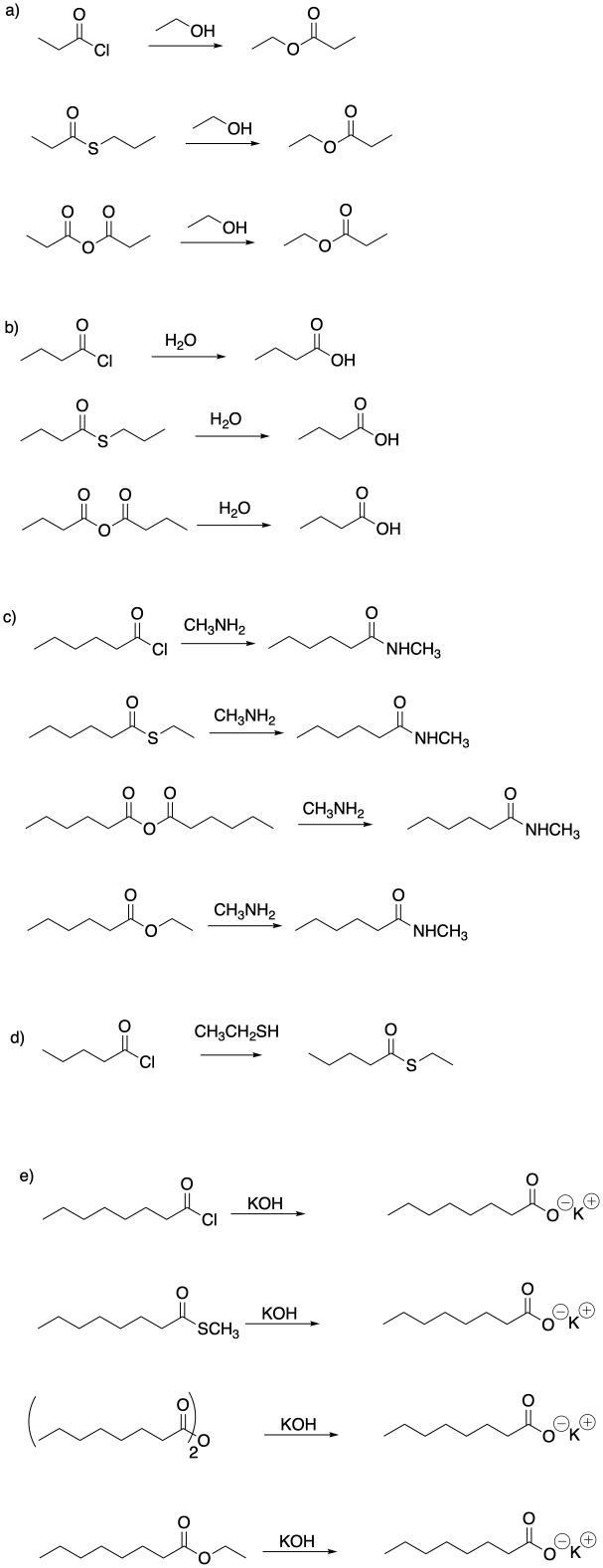
Problem CX4.2.
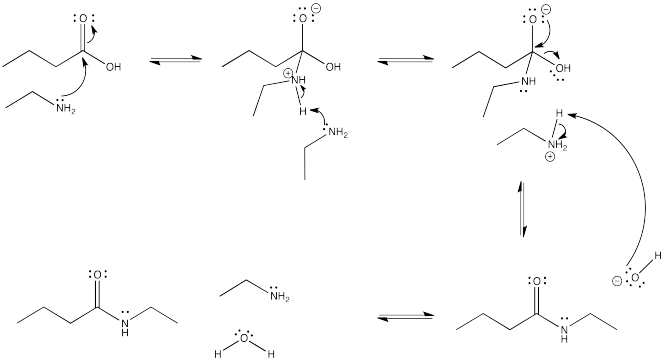
Problem CX4.3.
a)
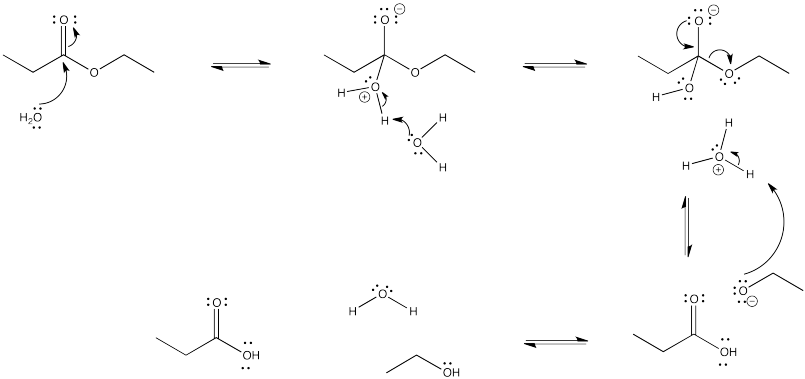
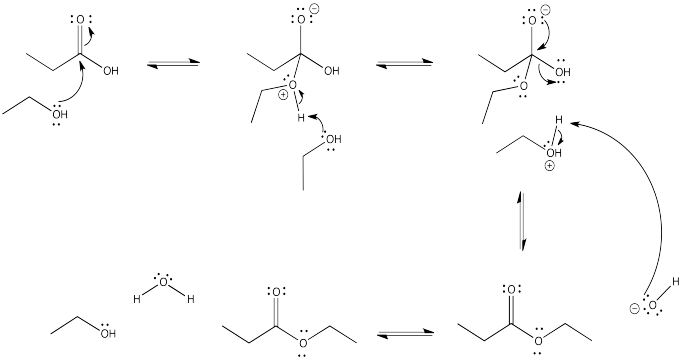
Problem CX4.4.

Problem CX4.5.

Problem CX4.6.

Problem CX4.7.
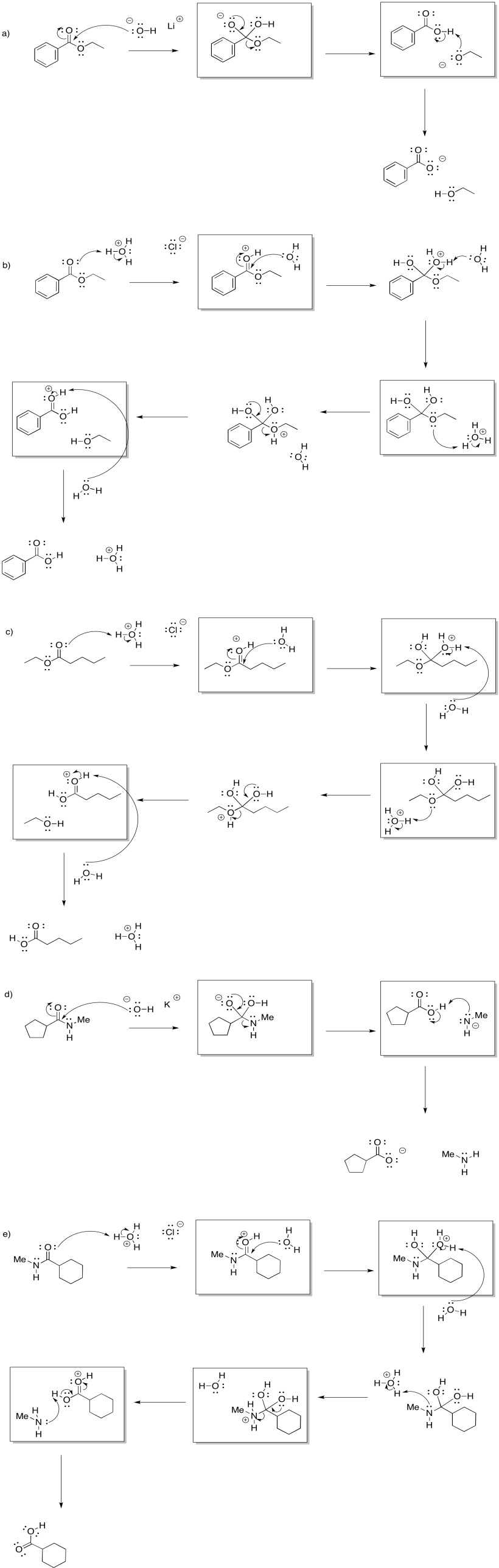
Problem CX4b.1.
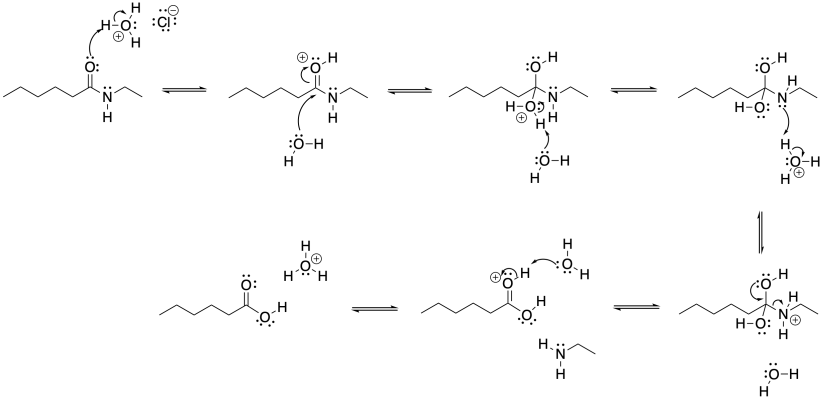
Problem CX4b.2.
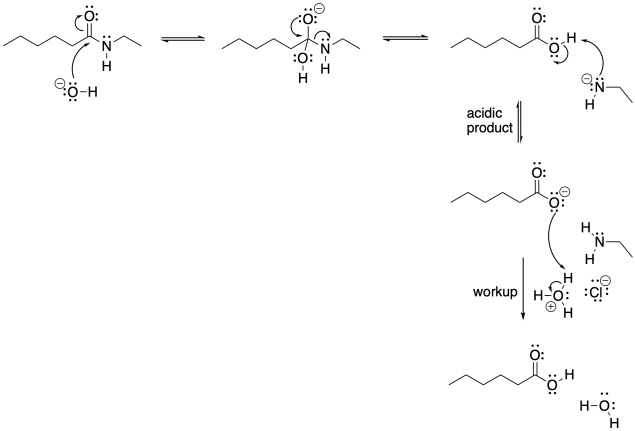
Problem CX5.1.
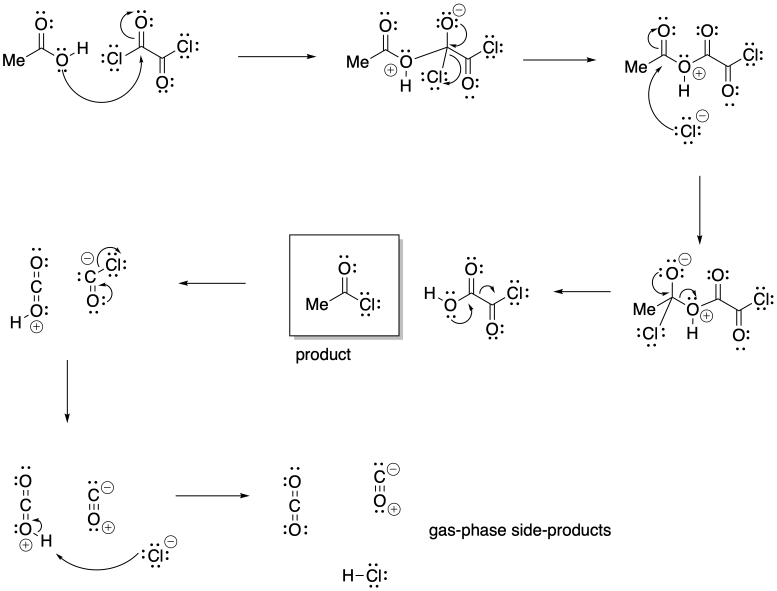
Problem CX5.2.
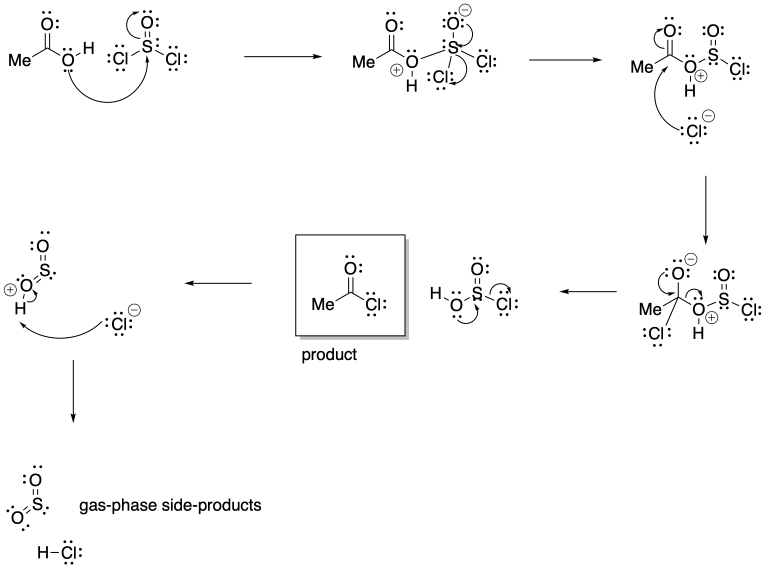
Problem CX5.3.
The release of gas phase products in either reaction pulls the equilibrium to the right. When these products escape the solution into the gas phase, le Chatelier's principle indicates more reactants will undergo reaction to maintain equilibrium.
Problem CX5.4.
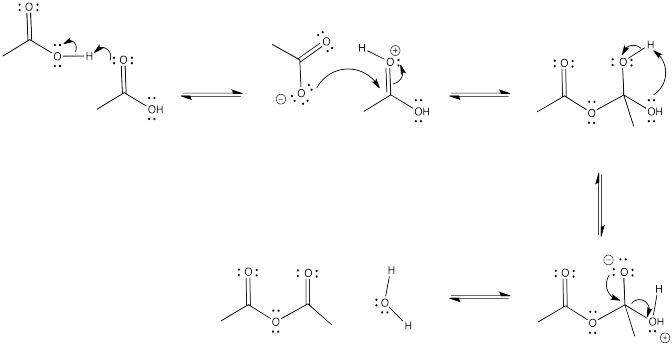
Problem CX5.5.
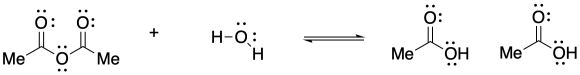
The reaction is favoured in the direction shown here. The anhydride is near the top of the ski hill and the carboxylic acid is closer to the bottom.
Problem CX5.6.
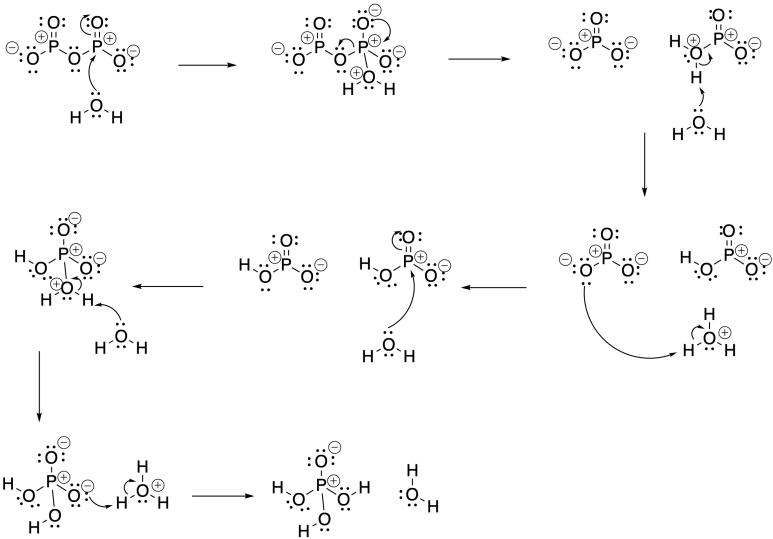
Problem CX5.7.
Phosphorus pentoxide consumes water as it is converted to phosphoric acid:
P2O5 + 3 H2O → 2 H3PO4
Addition of phosphorus pentooxide to the carboxylic acid will consume water and shift the equilibrium below to the right, producing the carboxylic anhydride:
2 CH3CO2H → (CH3CO)2O + H2O
Problem CX5.8.
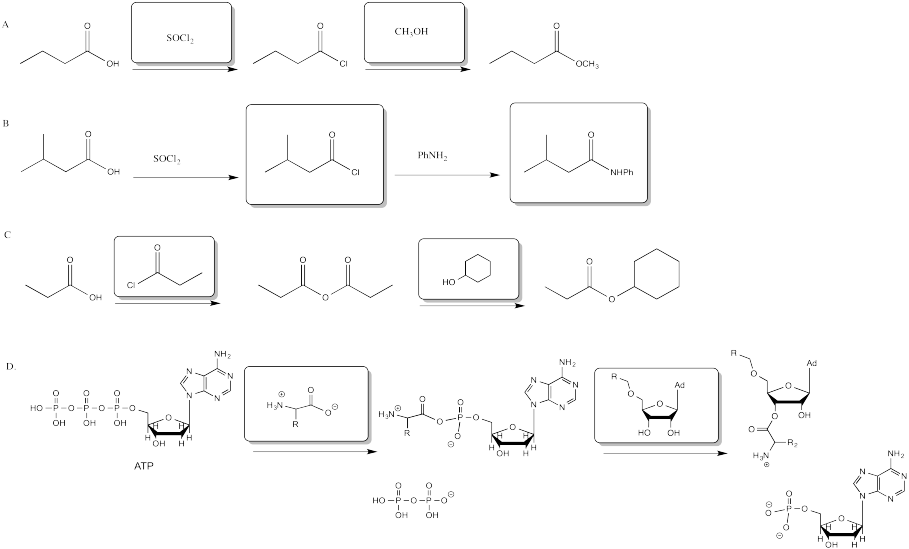
Problem CX6.1.
Because acid chlorides are at the top of the carboxyloid reactivity diagram (the ski hill), and other halides are likely to be similar in reactivity to the chloride, this reaction would be uphill from the other carboxyloids.
Problem CX6.2.
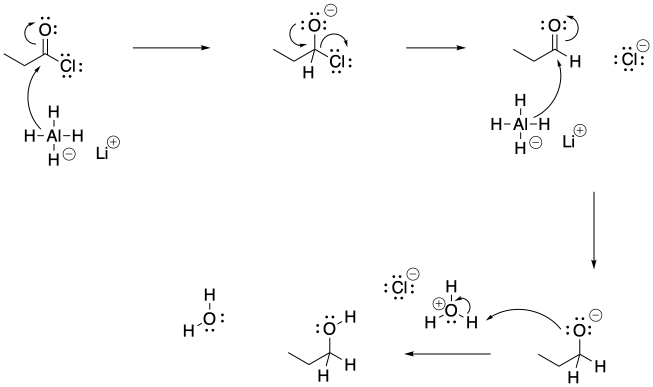
Problem CX6.3.

Problem CX6.4.
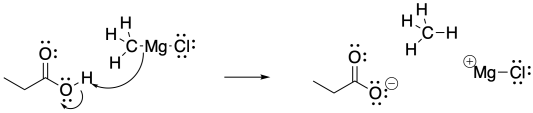
Because of the acidity of the carboxylic acid, the basic Grignard reagent is destroyed through protonation of the semi-anionic alkyl group.
Problem CX6.5.
Amides and carboxylates are the least reactive carboxyloids, so it might not be too surprising that they do not react with these nucleophiles.
Problem CX6.6.
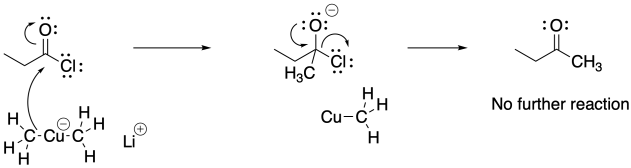
Acid chlorides typically react with these cuprate reagents. None of the other carboxyloids will typically react with these reagents and neither will aldehydes or ketones. As a result, organocuprate addition results in the formation of ketones, not alcohols.
Problem CX6.7.
a) Borohydrides could presumably react with acid chlorides, anhydrides and thioesters, which are the most reactive carboxyloids.
b) They probably can't react with amids or carboxylate ions, which are even farther downhill than esters.
Problem CX6.8.
a) If a hydride reacted with a carboxylate anion, the hydride would replace an oxide leaving group (O2-), forming an aldehyde. Aldehydes are much better electrophiles than carboxylate anions, so the aldehyde will quickly consume any lithium aluminum hydride and be converted to an alcohol.
b) Carboxylate ion is the least reactive carboxyloid. If lithium aluminum hydride can react with a carboxylate, then it can react with all of the other carboxyloids, too.
c)
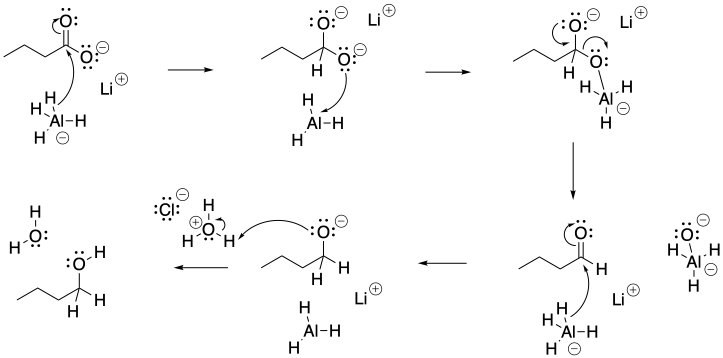
Problem CX6.9.
a) Lithium aluminum hydride is more reactive than sodium borohydride. The bond dipole between aluminum and hydrogen is much larger in AlH4- than in BH4- because the Pauling electronegativity value of aluminum is 1.61 compared to 2.04 in boron. The electronegativity difference is much greater with aluminum than with boron, placing more negative charge on the hydride in LiAlH4 than in NaBH4.
b) NaBH4 is more selective than LiAlH4.
c) More reactive reagents can react with anything, in the extreme case. Less reactive reagents can only react with more reactive partners. That means a less reactive reagent such as sodium borohydride is more selective than a more reactive reagent such as lithium aluminum hydride.
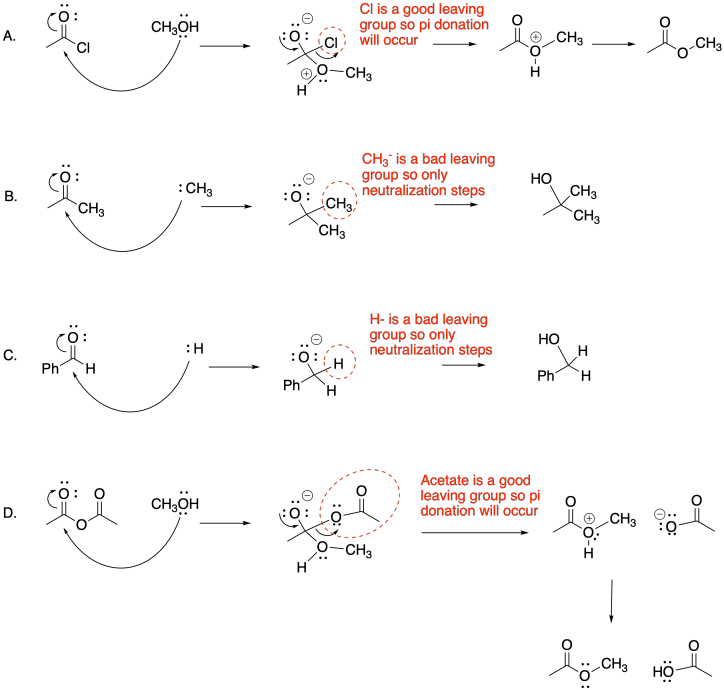
Problem CX6.10.a) Other examples displace the leaving group, which in this case would mean loss of the nitrogen group. In contrast, this case results in loss of the carbonyl oxygen.
b)
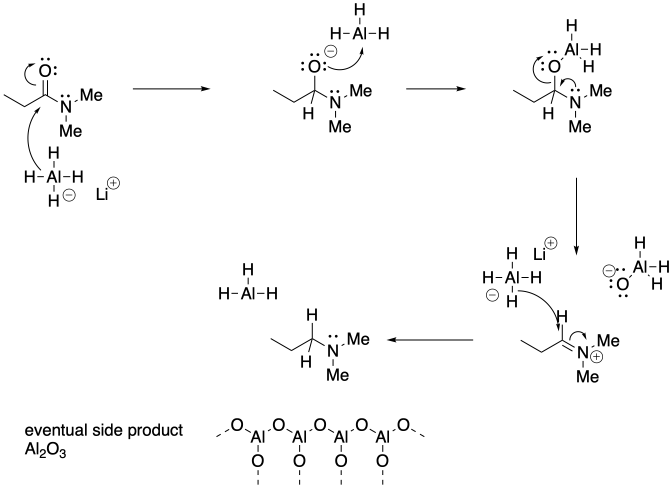
c) Nitrogen anion is not a very good leaving group, In this case, the loss of that group is not as likely, opening up another reaction pathway.
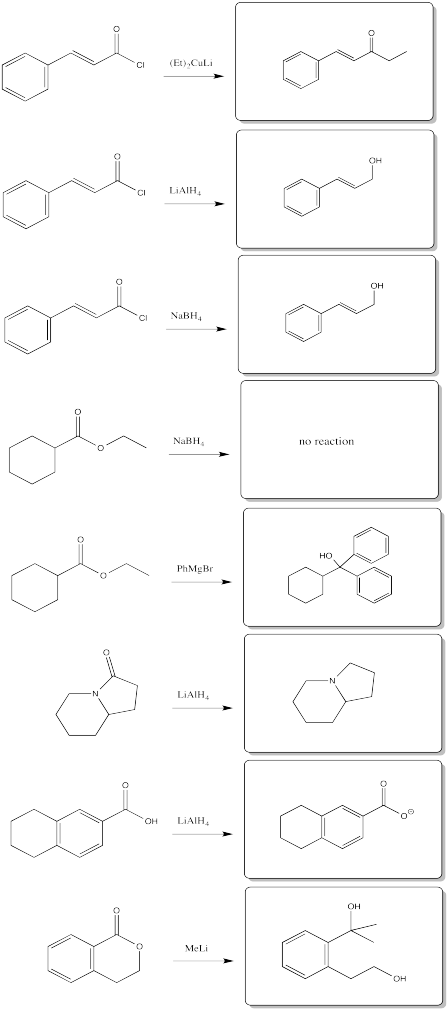
Problem CX6.11.
Problem CX7.1.
Problem CX7.2.
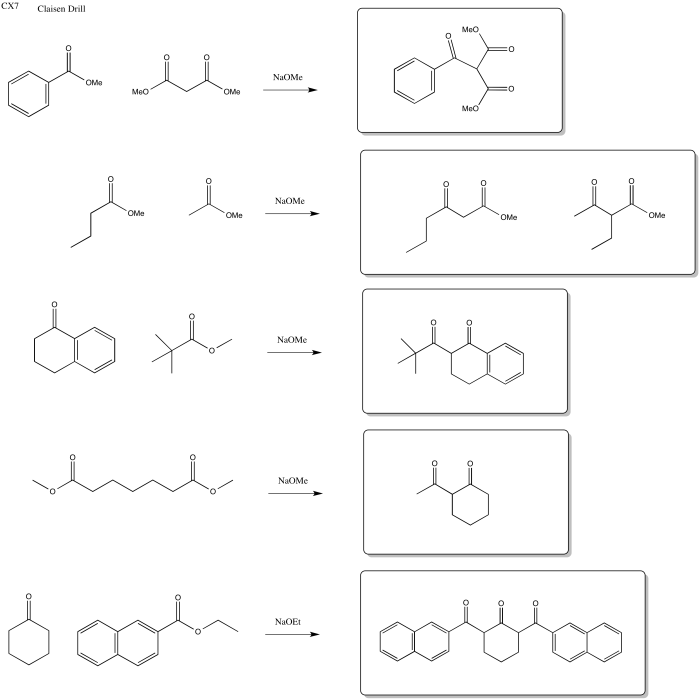
Problem CX7.3.
Problem CX7b.1.
a)
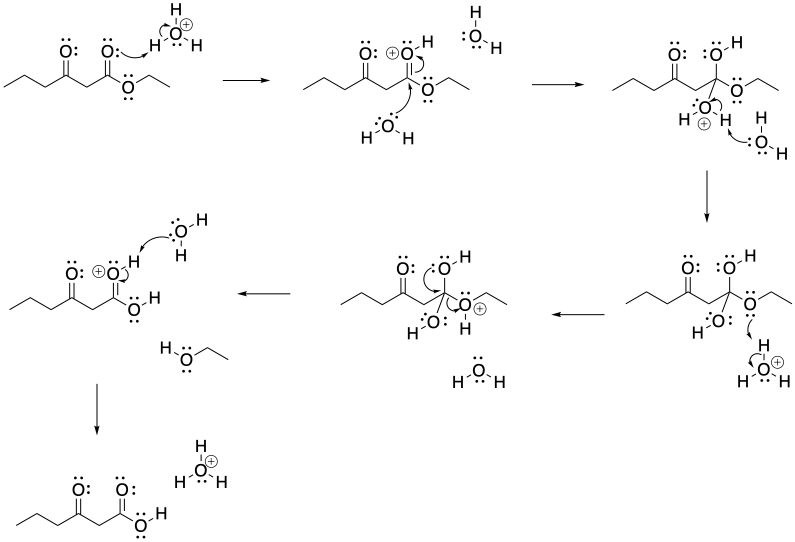
b)
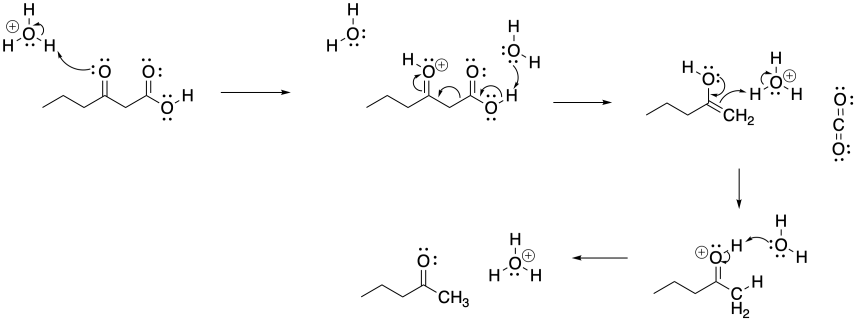
Problem CX7b.2.
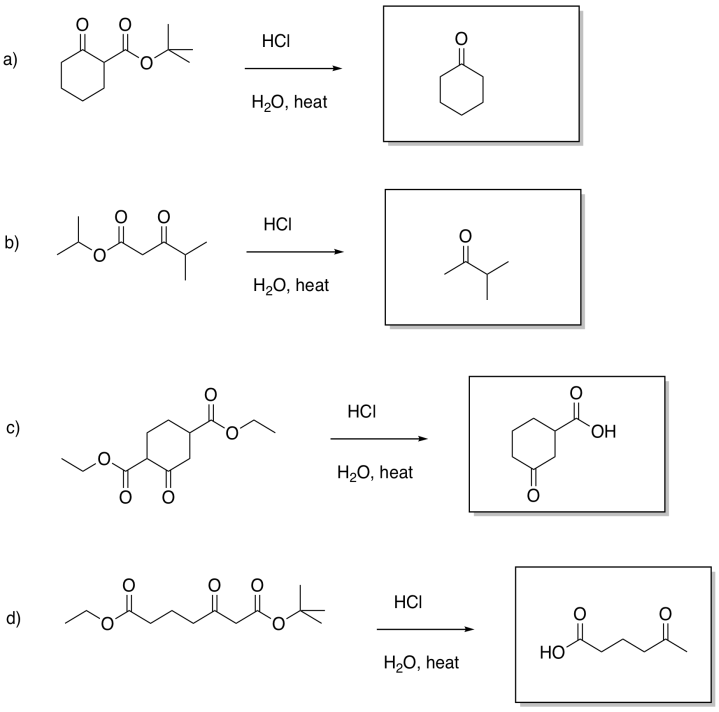
Problem CX8b.2.
i) In general, we compare a peak in the repeat unit (which occurs over and over) to a peak in an end group (which occurs only once in each chain) to find the number of repeat units or degree of polymerization. For example, if the chain were a dimer (n = 2) we would expect the integral for peak b to be twice as large as the integral for peak a.
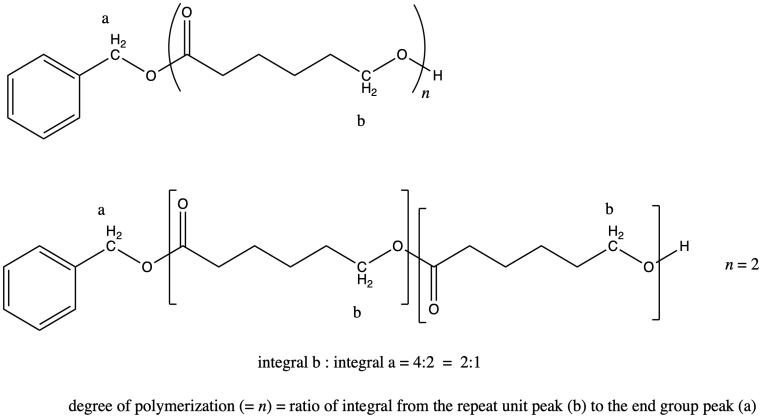
It works out that way because in each repeat unit, peak b represents two hydrogens, and in the end group, peak a represents two hydrogens. It's the same number of hydrogens in each position, so comparing the integration of the two peaks tells you directly how many repeat units there are.
If that were not true (if peak b represented only 1H and peak 1 represented 2 H), then we would have to factor that difference into the answer.
The question states that the integration ratio of b:a is 50:1, so that means the degree of polymerization = 50.
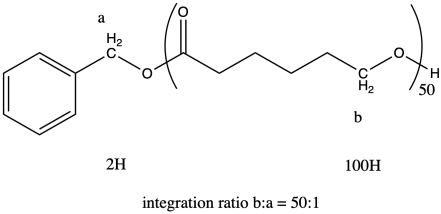
The molecular weight is therefore about 50 times the molecular weight of the monomer (114.4 g/mol), or 5,720 g/mol (sometimes expressed as 5,720 Da; a Dalton is just 1 g/mol). There are also end groups (from benzyl alcohol) that contribute a little weight, so the total molecular weight is 5,720 + 108 g/mol = 5,828 g/mol.
ii. Once again, peak a represents two hydrogens, and so does peak b (although the peak b hydrogens occur at two different places in the molecule and differ stereochemically from each other). That means that, once again, the ratio of integrals of peak b to peak a tells us the degree of polymerization. If the ratio were 3:1, we would have n = 3.
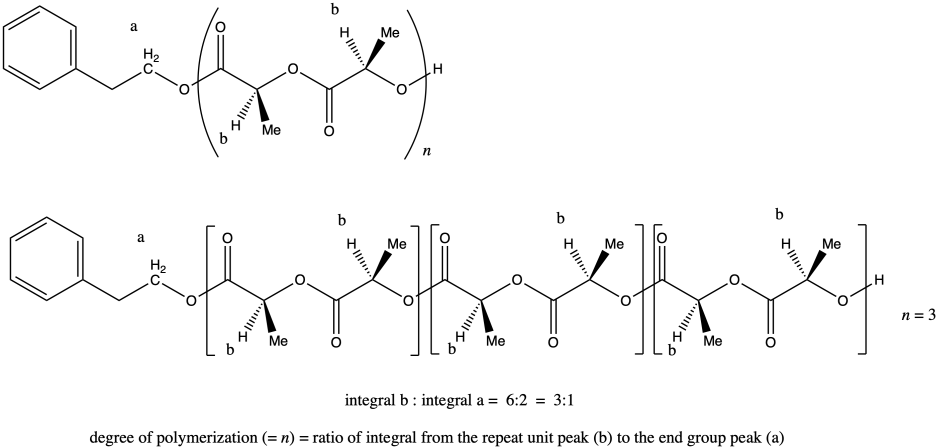
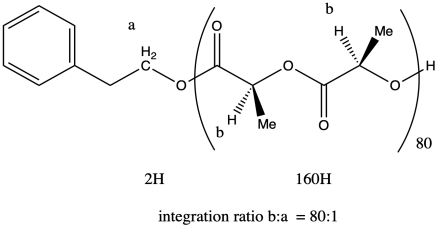
The molecular weight is therefore about 50 times the molecular weight of the monomer (144.1 g/mol), or 11,530 g/mol. Adding in the end groups (from benzyl alcohol), the total molecular weight is 11,530 + 108 g/mol = 11,638 g/mol.
Note that in certain diastereomers of lactide (LLA or DLA) we could actually draw the repeat unit as a C3H4O2 unit rather than as the larger C6H8O4 unit, but in this case the two chiral centers are actually different, so we have to keep the larger repeat unit to show the presence of both stereochemical configurations.
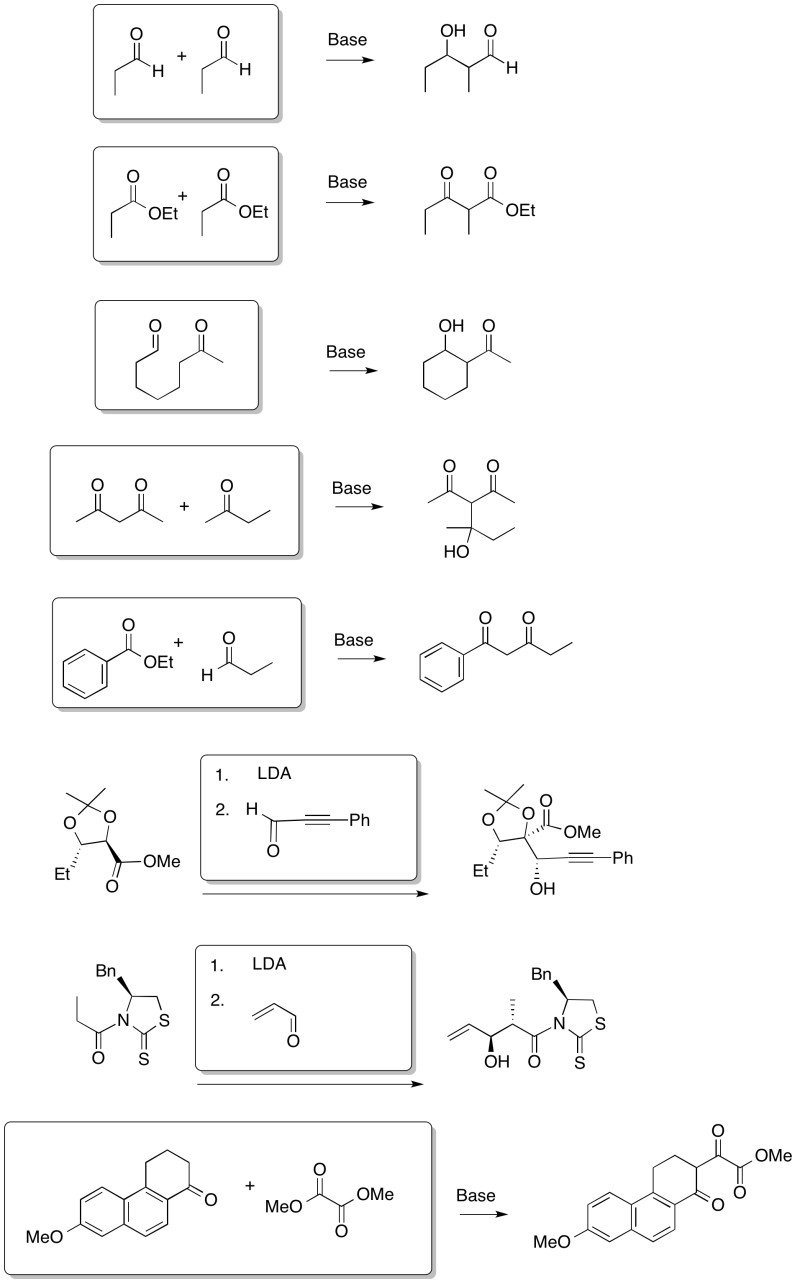
Problem CX9.1.
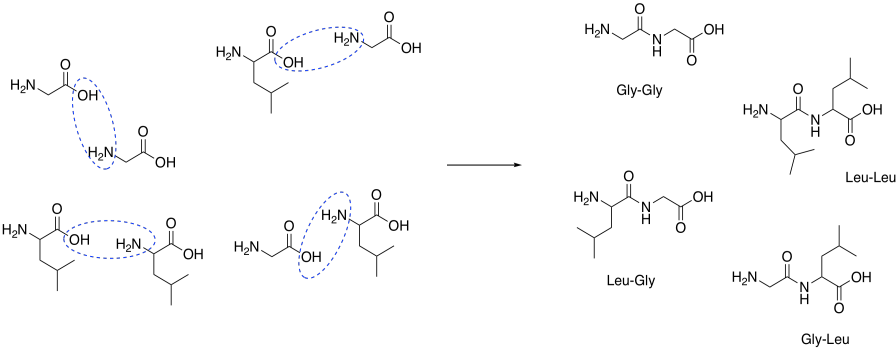
Problem CX9.2.
That combination would give thw following dipeptides:
ala-ala ala-gly ala-val
gly-gly gly-ala gly-val
val-val val-ala val-gly
Of course, we might also get tripeptides, such as ala-ala-gly-val, and so on.
Problem CX9.3.
Carboxylic acids usually require activation before they can act as nucleophiles. That problem is actually complicated here because the carboxylic acid is in equilibrium with a carboxylate salt (read further on the page).
Problem CX9.4.
The relative reactivity of carboxyloids results from a balance between sigma electron withdrawing effects and pi-donation. An electronegative atom attached to a carbonyl tends to withdraw electron density, making the carbonyl even more positive and electrophilic. On the other hand, pi-donation from a neighbouring atom with a lone pair actually lowers electrophilicity by forming a stable, conjugated system.
In a carbamate, an additional electronegative atom is added to the carbonyl: it has an oxygen as well as a nitrogen adjacent to the C=O group. That atom draws electron density away from the carbonyl, making it more electrophilic. However, pi-donation from the additional oxygen does not result in a more stable conjugated system. The maximum pi system is still just three atoms long; it either involves conjugation of the O-C=O or the N-C=O unit. It does not, for example, lead to an even more stable conjugated system that is four atoms long.
As a result, the added oxygen probably contributes more to the electron-withdrawing effect than it does to stabilisation of the pi system.
Problem CX9.5.
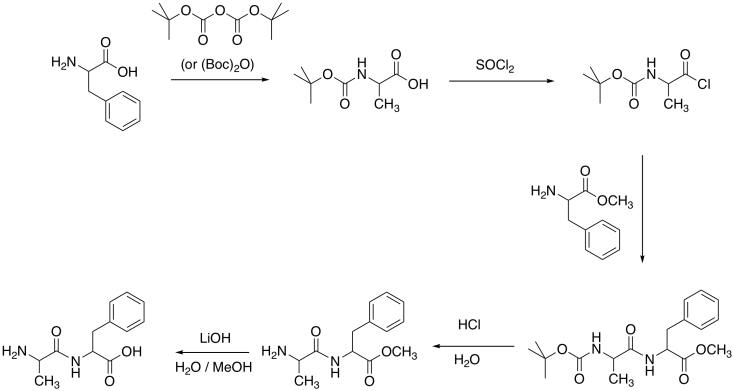
Problem CX9.6.
The more polar hydrochloride salt of EDCI is often used, as pictured. This more polar compound dissolves well in polar solvents, such as water, that also dissolve amino acids.
One can also imagine the amino group in EDCI acting as a site for catalysis, shuttling protons from one place to another.
Problem CX10.1.
This change in charge results because, although amines are easily protonated, amides are not. Protonation of an amide would result in a cation adjacent to the very positive carbonyl carbon, leading to a buildup of localized positive charge. That wouldn't be easy. Furthermore, the amide nitrogen is not very likely to donate its electrons to a proton in the first place. Its protons are too busy. They are tied up in conjugation with the carbonyl, so they really aren't available to act as the lone pair of a base.
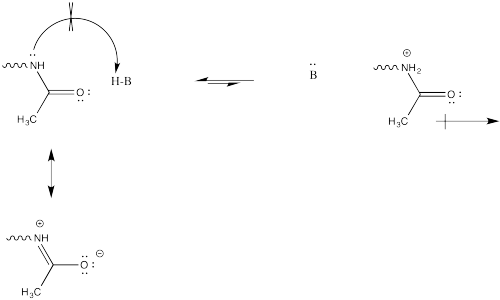
Problem CX12.1.
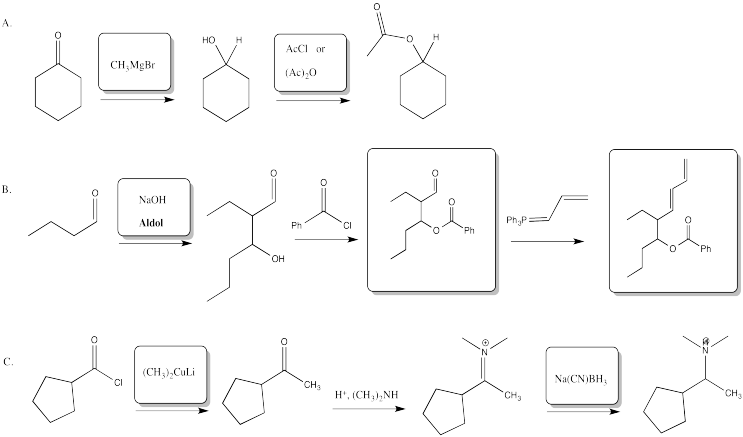
Problem CX12.2.
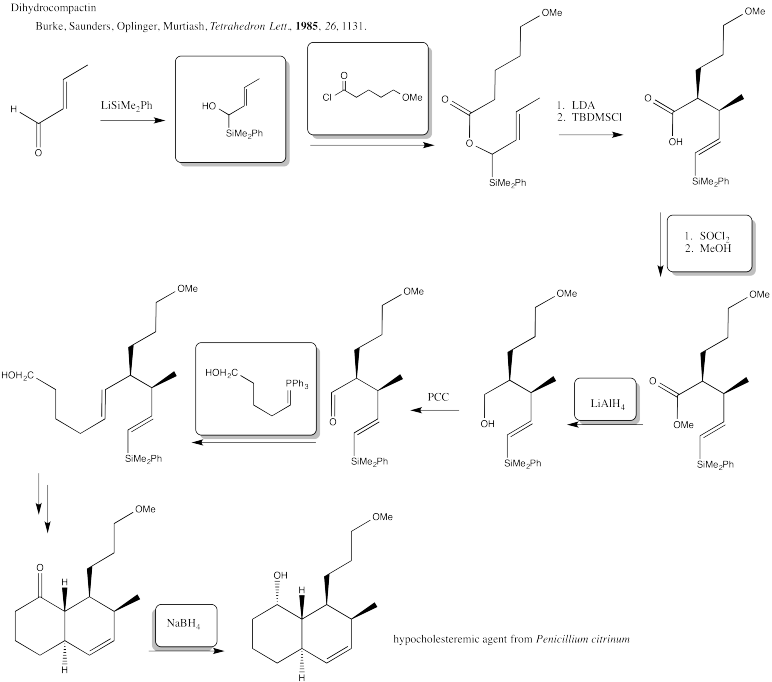
Problem CX12.3.
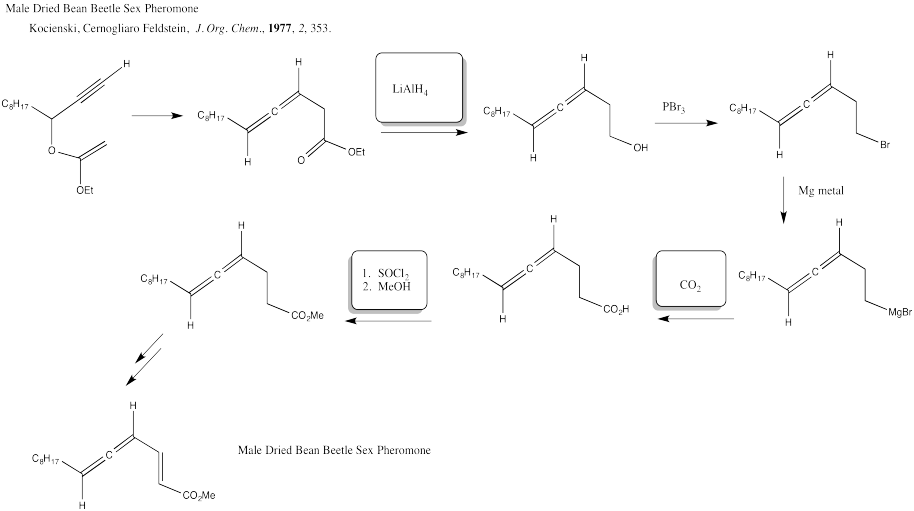
Problem CX12.4.
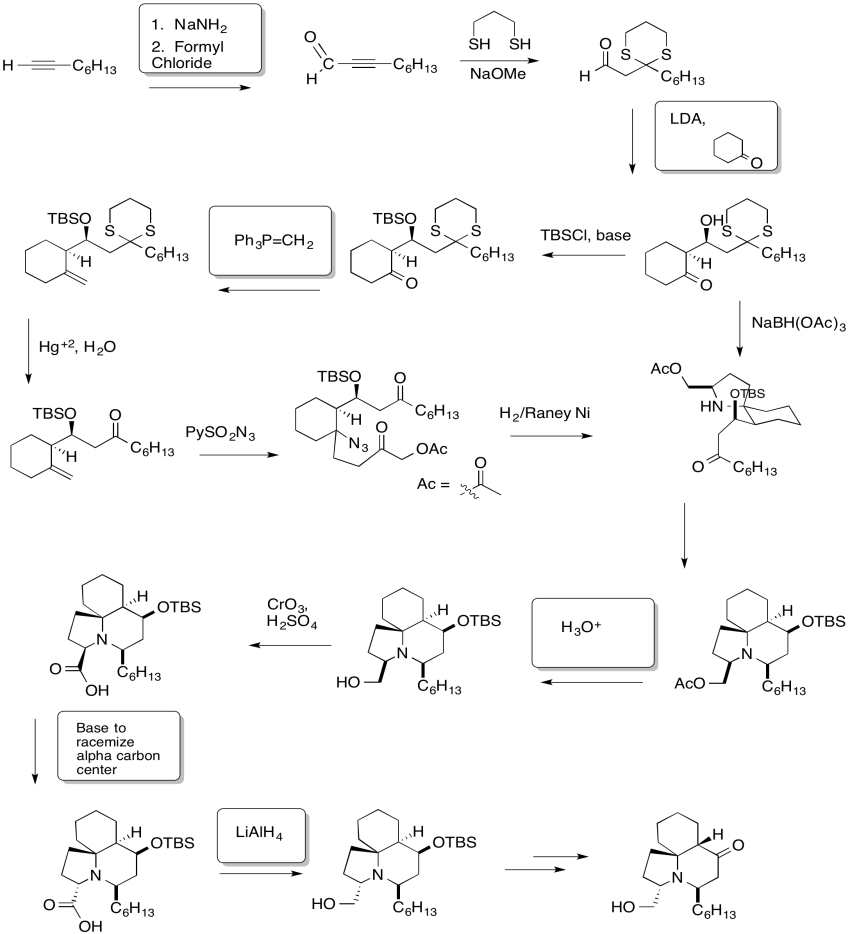
Problem CX12.5.
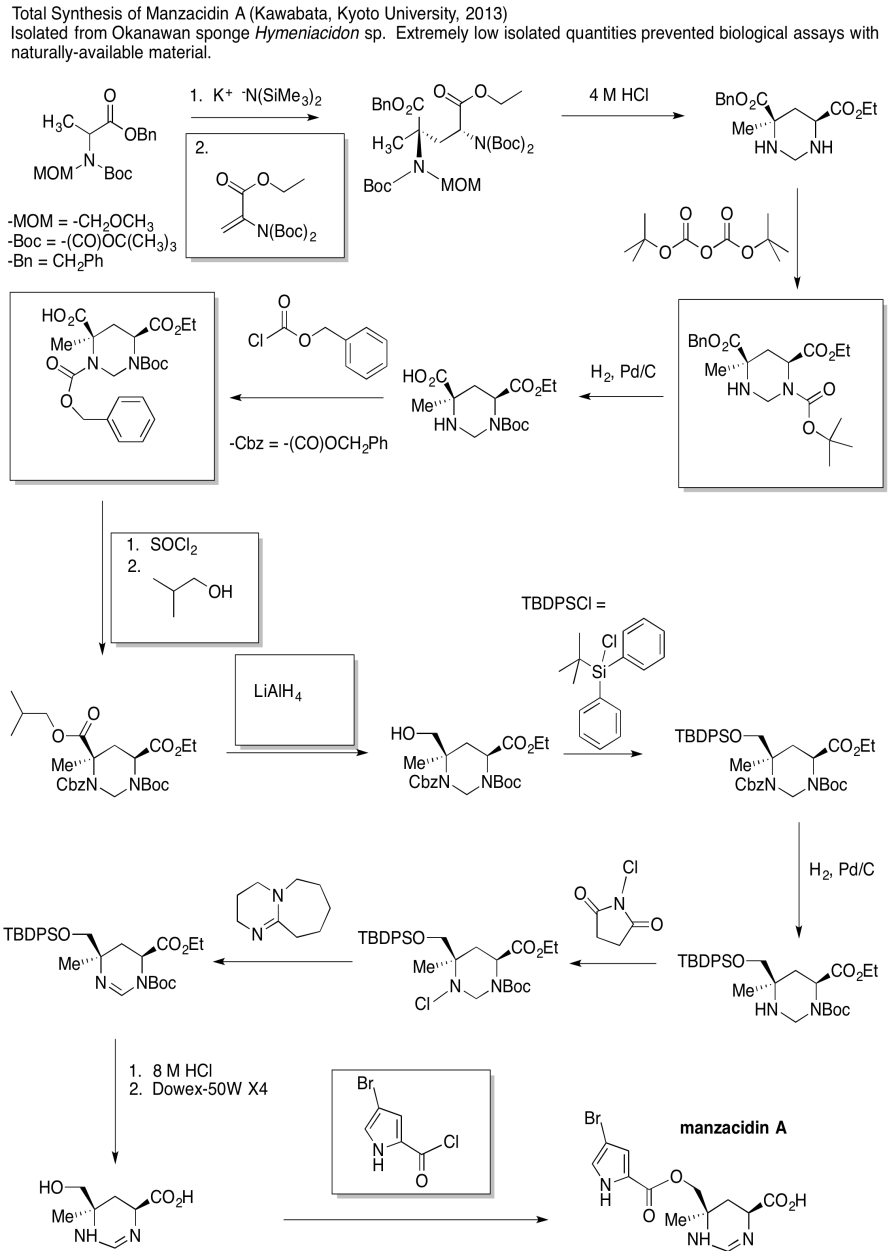
Problem CX12.6.
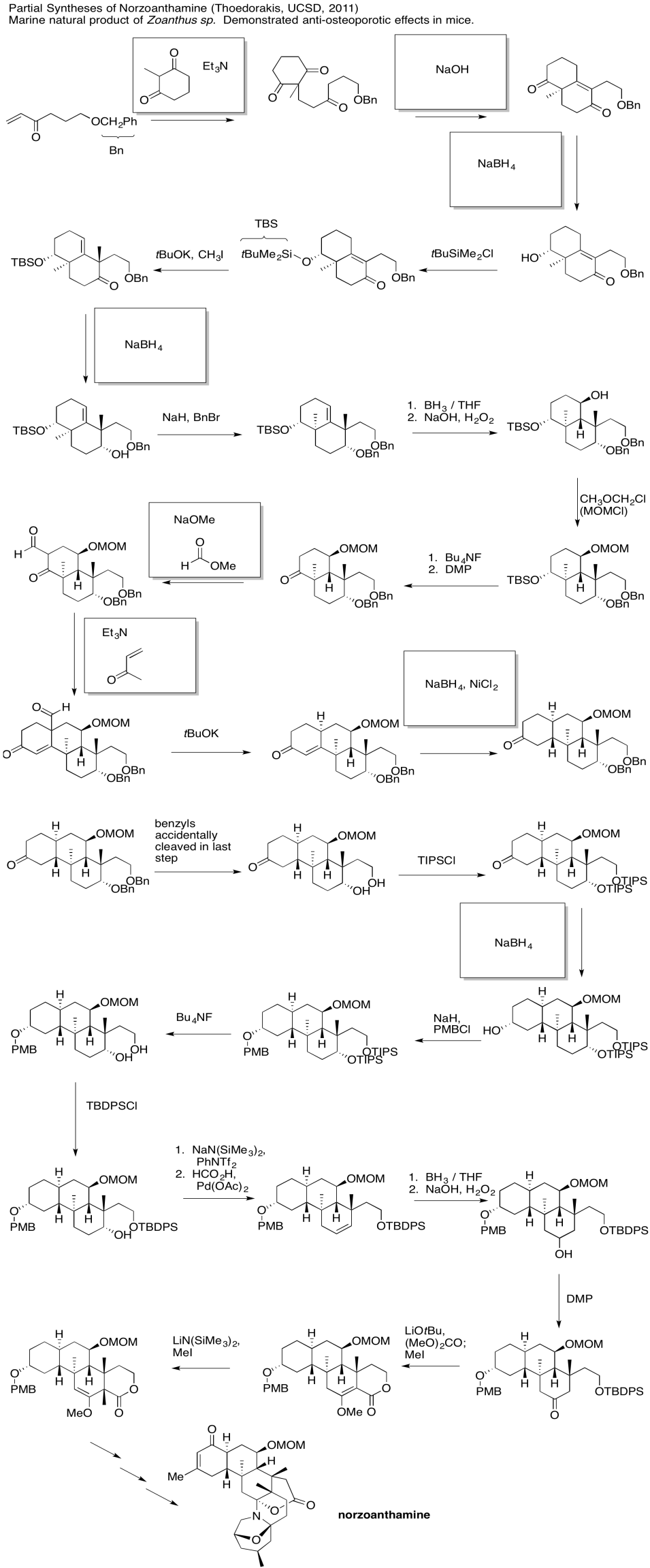
Problem CX12.7.
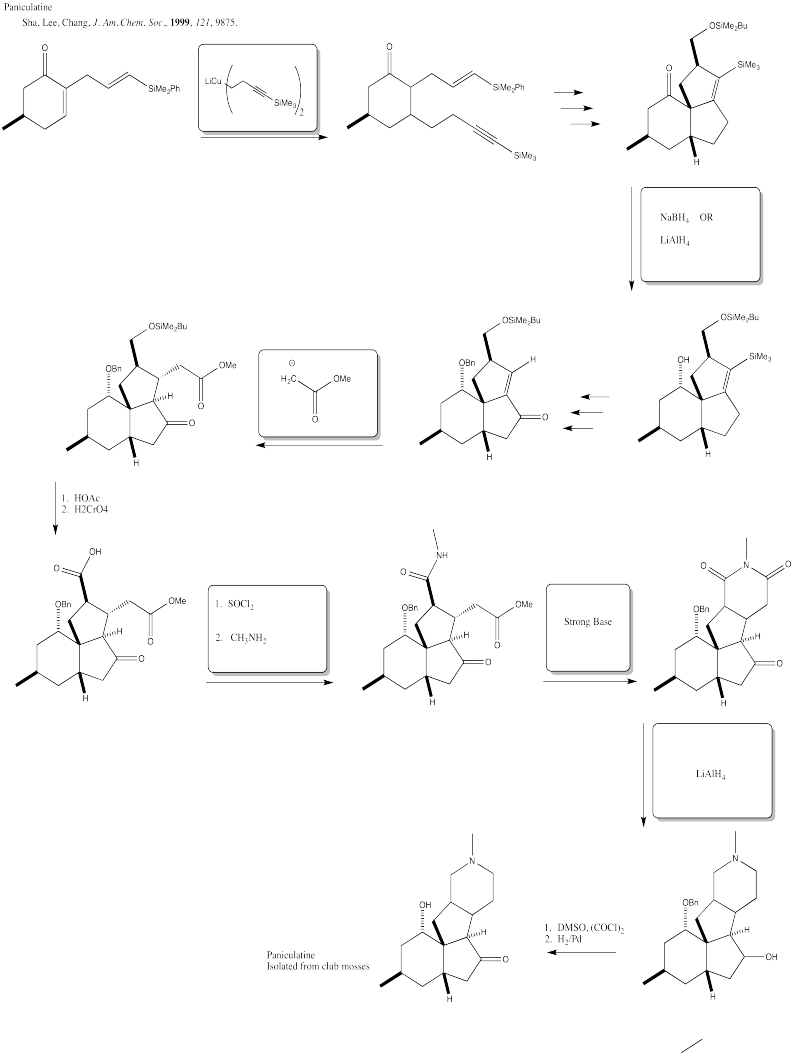
Problem CX12.8.
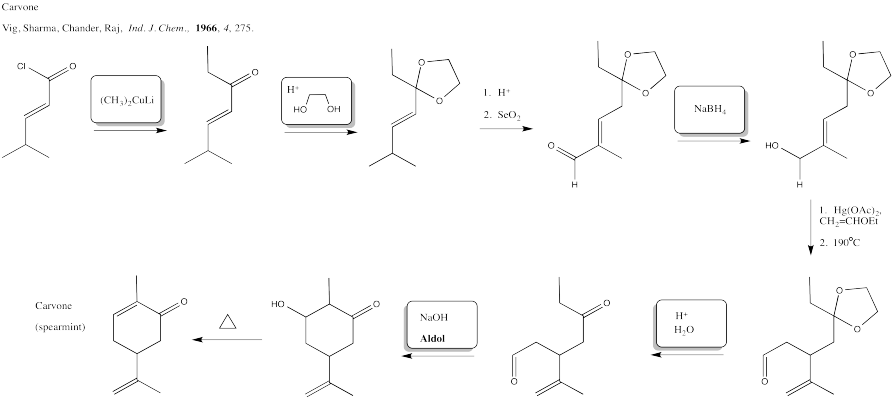
Problem CX12.9.
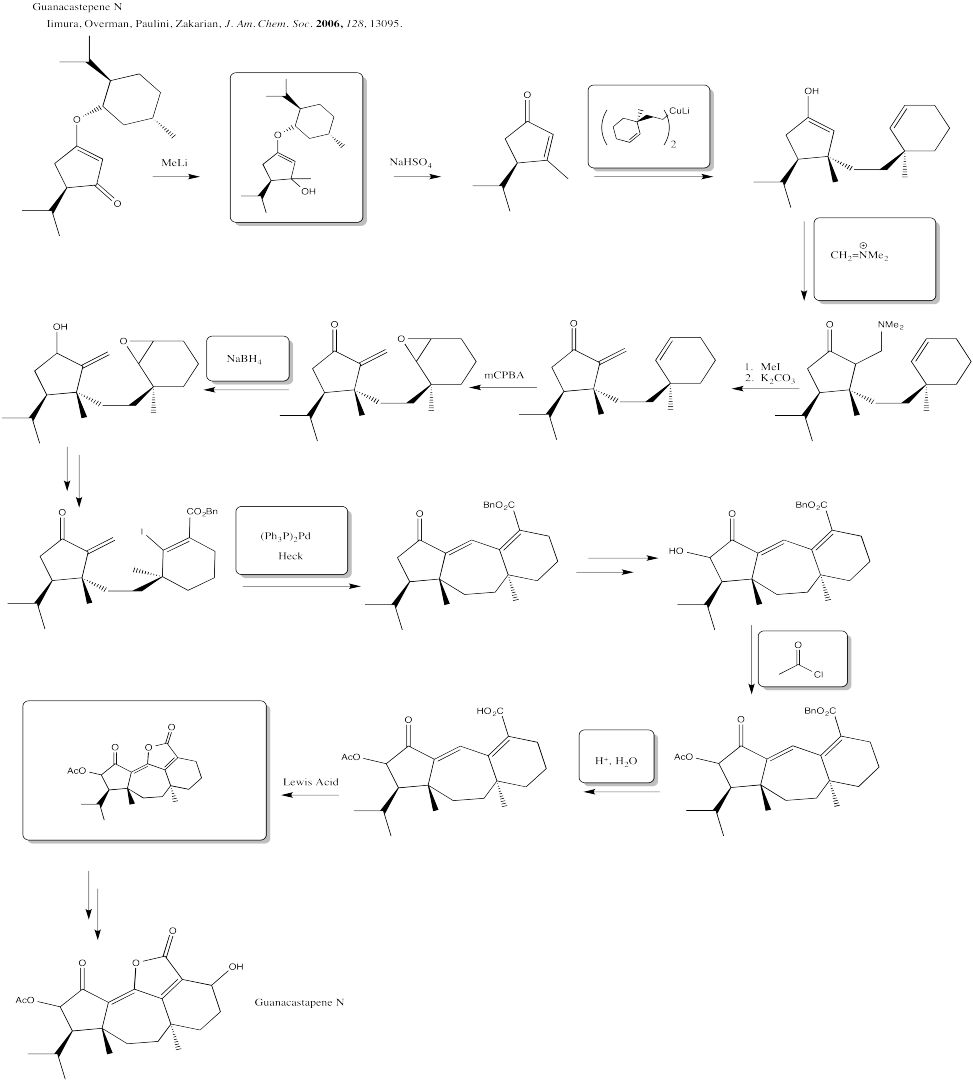
Problem CX12.10.
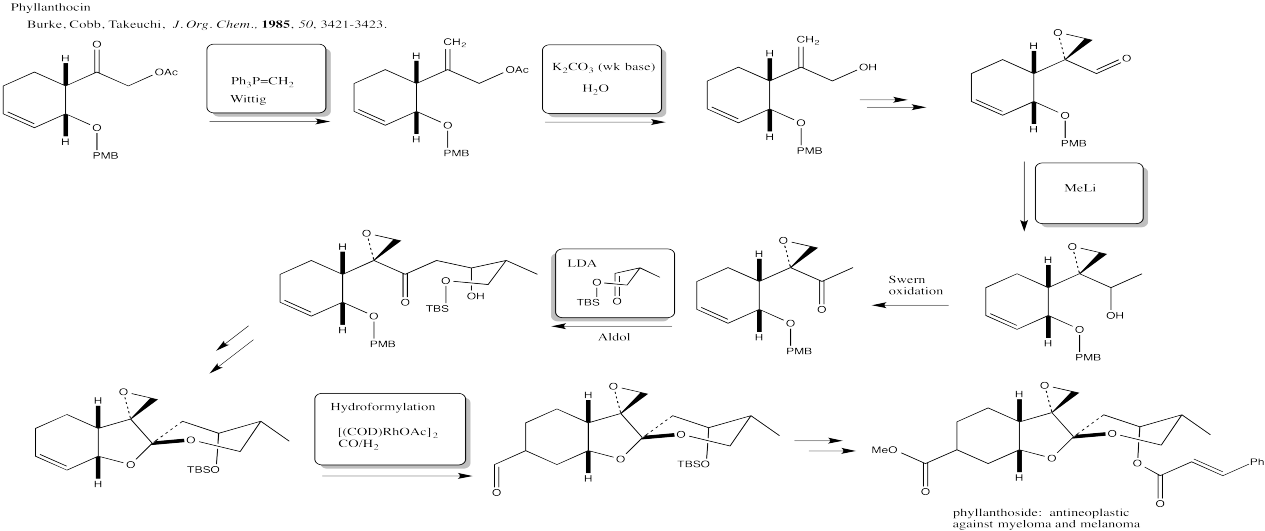
Problem CX12.11.
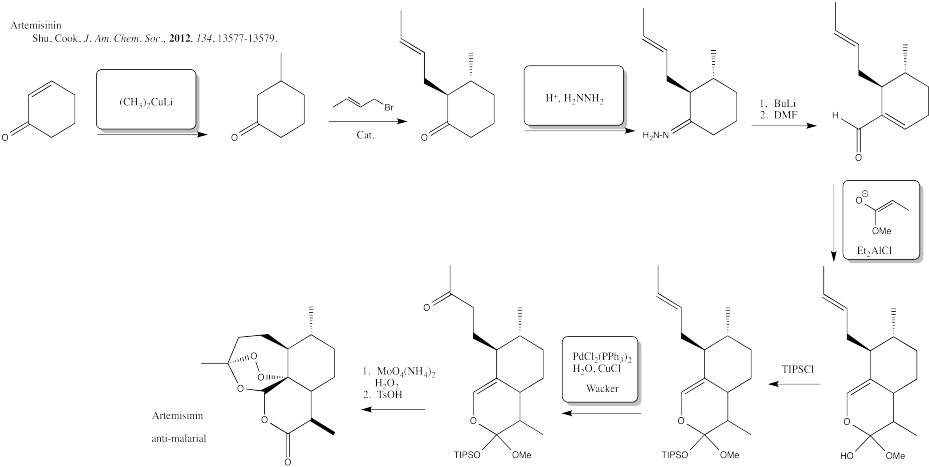
Problem CX12.12.
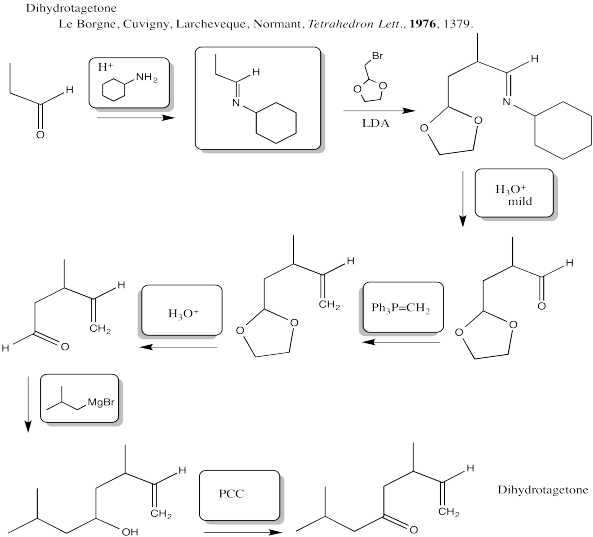
Problem CX12.13.
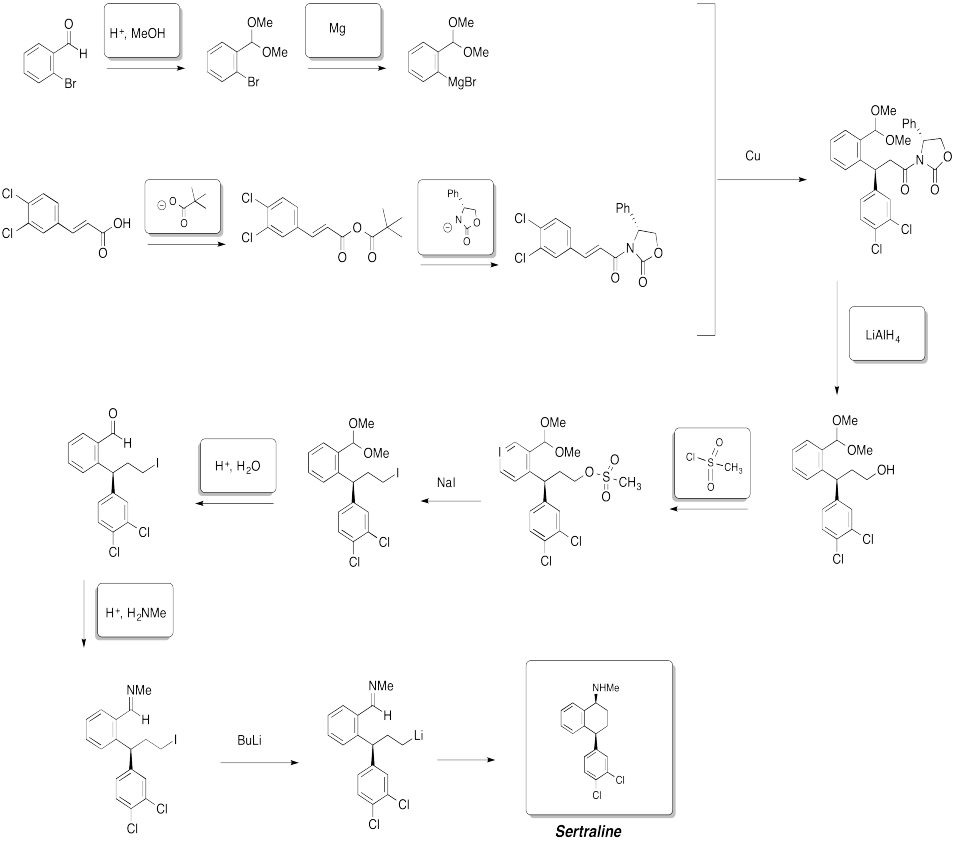
Problem CX12.14.
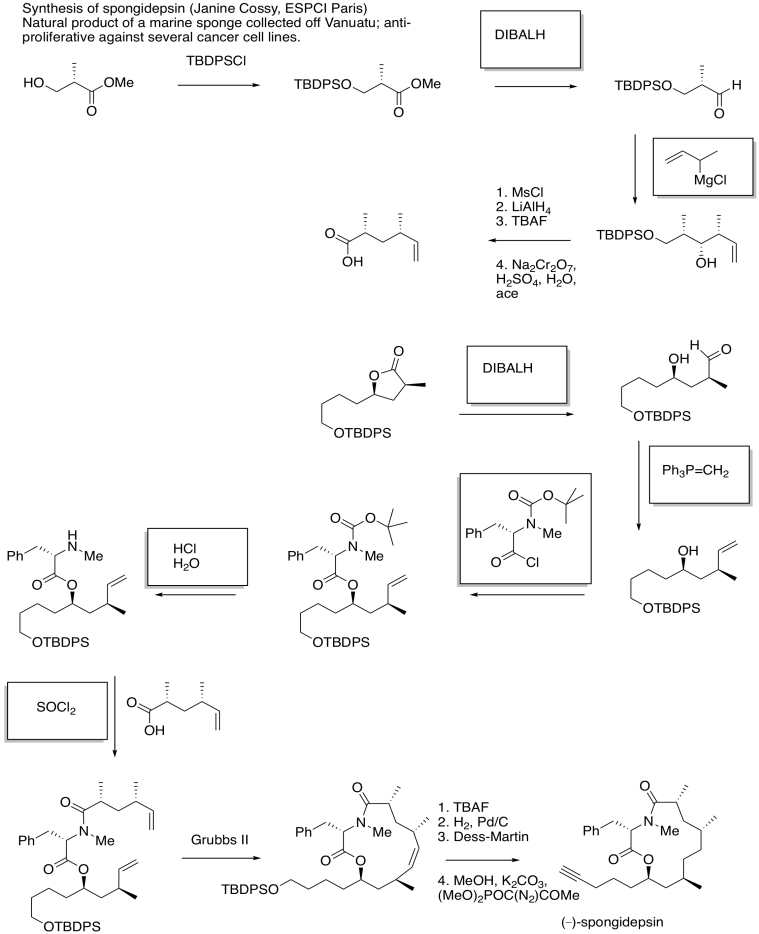
Problem CX12.15.
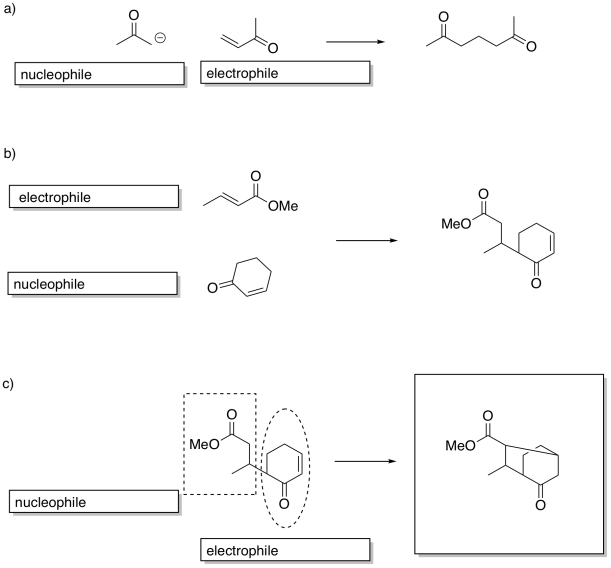
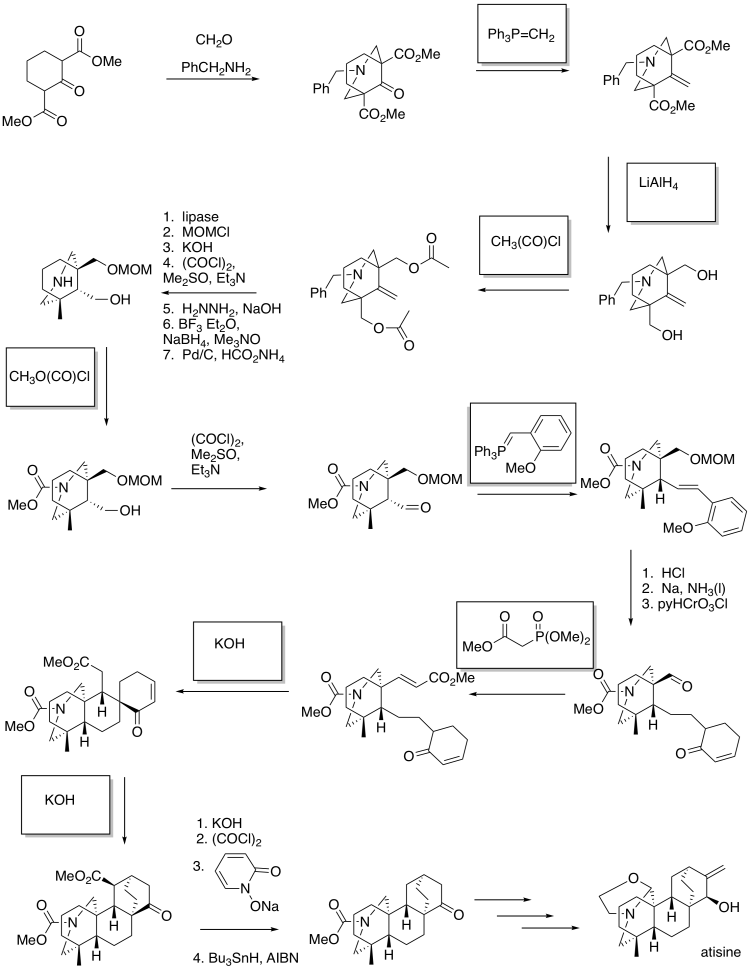
Problem CX12.16.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carboxyl Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry