Reactivity in Chemistry
Aliphatic Nucleophilic Substitution
NS17. Nucleophilic Substitution in Synthesis: Amines
Amines can be synthesized through nucleophilic substitution. Using an alkyl halide and the proper nucleophile, the halide can be replaced by an amino group.
![]()
Figure NS17.1. What nucleophile would turn an alkyl halide into an amine?
If an amide ion were used as the nucleophile, elimination would be a pretty sure thing. An amide ion is even more basic than a hydroxide ion. The nitrogen atom is less electronegative than the oxygen atom of hydroxide. This was the same problem with making alcohols and ethers with hydroxide or alkoxide ions, but now the problem is more severe.
![]()
Figure NS17.2. An amide ion (NH2-) is too basic to make an amine.
In the case of alcohol and ether syntheses, one approach was to simply use a neutral form of the nucleophile rather than an anion. We could do that in this case. Ammonia still has a lone pair and it is a pretty good nucleophile. We don't need a negative charge on the nitrogen for it to displace a halogen from an alkyl halide.
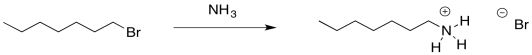
Figure NS17.3. Ammonia is nucleophilic enough to make an amine.
Because nitrogen is a litle less electronegative than oxygen, ammonia is a better nucleophile than water. This substitution works even better than the substitution of water for a halide. However, there are other problems.
Ammonia is nucleophilic, but it is also basic. That can be helpful. In the synthesis of alcohols and ethers, addition of a neutral nucleophile had to be accompanied by a weak base, otherwise the buildup of acid in the reaction might cause unexpected side reactions, including the breaking of ether linkages that you were trying to make. However, it means that half of the ammonia that you put into the reaction would get used up in acid-base reactions. You would need to add twice as much ammonia as alkyl halide. One molecule of ammonia would act as the nucleophile in each reaction, and one would act as the base.

Figure NS17.3. Ammonia is basic enough to deprotonate a cation.
Remember, the order of steps does matter here. Sure, you could imagine some miniscule equilibrium in which one ammonia has plucked a proton off another, but a quick look at a pKa table tells you that's not very likely. The proton will only be removed after the first ammonia has donated its lone pair and the neutral nitrogen atom becomes part of a much more acidic ammonium ion.
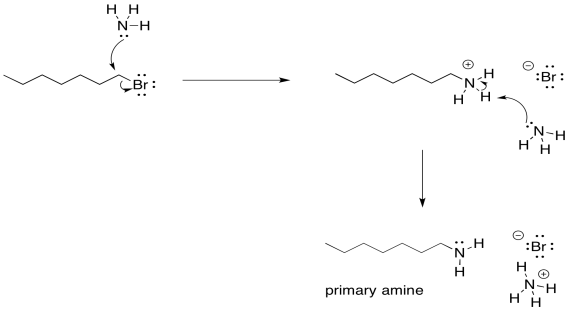
Figure NS17.4. Mechanism of amine formation using an alkyl halide and ammonia.
There is another problem, though. Once that ammonium ion has been deprotonated, the nitrogen gets its lone pair back again. It becomes nucleophilic again. Because nitrogen is pretty nucleophilic, there is nothing stopping this newly-formed amine from reacting with another alkyl halide. It will do so, pretty reliably. That will lead to formation of some secondary amine, in addition to the primary amine that you may have been aiming for. A secondary amine has two alkyl groups attached to the nitrogen, rather than just one.
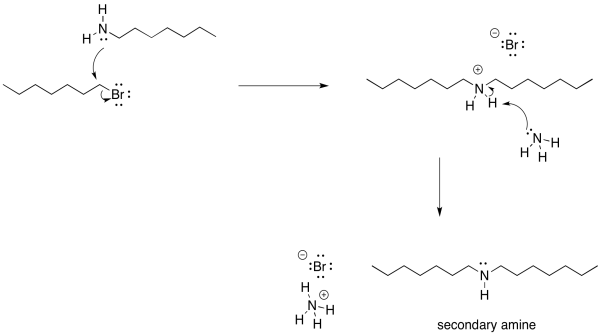
Figure NS17.5. The problem of multiple alkylation: the reaction does not stop at the primary amine.
Of course, now that we have started down that road, there's no going back. As soon as that secondary ammonium ion is deprotonated, it gets a lone pair restored. Once it has a lone pair, it becomes pretty nucleophilic. It will donate to another alkyl halide and form a tertiary ammonium ion. That tertiary ammonium ions will get deprotonated almost immediately.
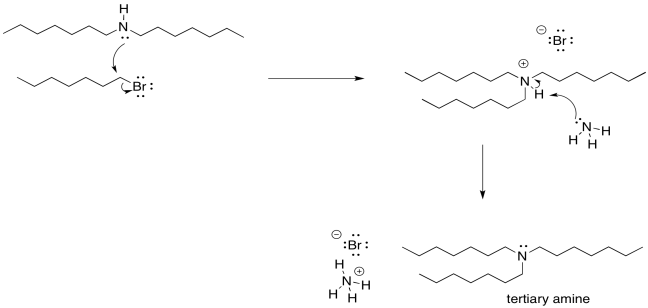
Figure NS17.6. The problem of multiple alkylation: the reaction does not stop at the secondary amine.
As soon as the tertiary amine forms, of course it is just going to do the same thing. It will donate its lone pair to an alkyl halide. It forms a quaternary ammonium ion. This time, however, there is no proton on the nitrogen. It can't easily be deprotonated. It remains as a quaternary ammonium salt. Quaternary ammonium salts are really quite stable; they are used in household consumer products all the time. Surfactants (like those used in cleaning sprays) and anti-static agents (which might show up in dryer sheets and shampoos) frequently use quaternary ammonium salts.
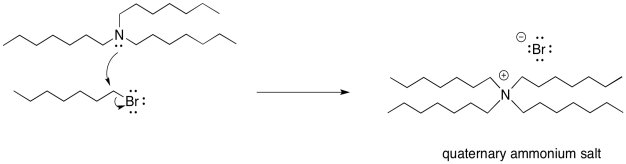
Figure NS17.7. The problem of exhaustive alkylation: the reaction does not stop at the tertiary amine.
Now, let's think about our ratio again. Suppose we started with that 2:1 ratio of ammonia to alkyl halide, knowing that as soon as a first ammonia molecule bonded with an alkyl, a second ammonia would immediately take a proton. By forming a quaternary ammonium salt, we have actually used up four alkyl halides with one ammonia nucleophile. We have also used up three other ammonia molecules as bases. We have used the reactants up in a 1:1 ratio and we will have half of the ammonia left over.
So it sounds like a 2:1 ratio of ammonia to alkyl halide might get us a primary amine, but a 1:1 ratio might get us to a quaternary ammonium salt. What if we had stopped at a secondary amine? Then we would use up two alkyl halides as electrophiles, one ammonia as a nucleophile, and two ammonias as bases. That's a 3:2 ratio of ammonia to alkyl halide, or 1.5:1. Similar analysis would tell you that stopping at a teriary amine would require a 1.3:1 ratio of ammonia to alkyl halide.
The point is, formation of these different amines require ratios of ammonia to alkyl halide that are really very similar. Realistically, there is no way we could add these exact ratios of reagents to a reaction and expect things to stop at the right place. Remember, a reaction is taking place with millions of molecules at once, and probability says that all four pathways will be followed by significant fractions of the reactants. All of these ratios of ammonia to alkyl halide will lead to the same thing: a mixture of all possible products. There would invariably be some leftover amine or leftover alkyl halide, too, once the other reagent had run out.
Practically speaking, there are only two products that you can make from this reaction. You could make a primary amine if you used a vast excess of ammonia, so that you ensured that any alkyl halide was much more likely to run into an ammonia molecule long before it ran into an amine molecule.

Figure NS17.8. Reactant ratios can sometimes be used to control the reaction.
In this case, you would have leftover ammonia. That should be pretty easy to remove because of its low boiling point.
Alternatively, you could make a quaternary ammonium salt if you used a large excess of alkyl halide.
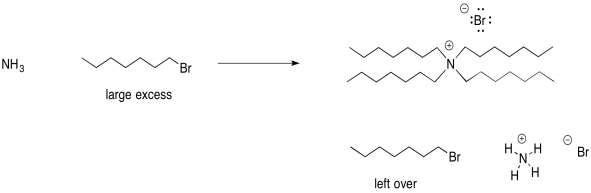
Figure NS17.9. Reversing the reactant ratio changes the product.
In this scenario, you would have leftover alkyl halide. The difference in solubility between the alkyl halide and the quaternary ammonium salt could help separate these two materials.
You might recall that in the section on the synthesis of alcohols and ethers, an alternative strategy used a resonance-stabilised, anionic oxygen nucleophile to make alcohols. The same strategy is often used with the synthesis of amines. This approach is called a Gabriel amine synthesis.
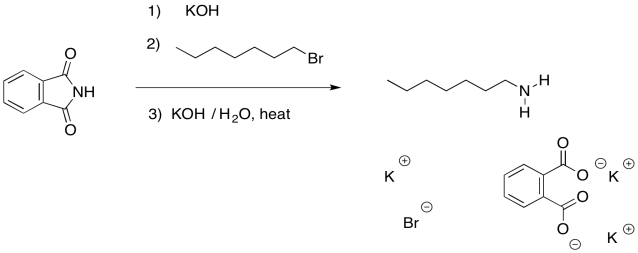
Figure NS17.10. The Gabriel synthesis of amines.
The nucleophile in this case is a phthalimide ("FTAL-im-id" or "FTAL-im-eyed") ion. The phthalimide ion is easily formed by a strong base such as potassium hydroxide, becaus the anion obtained is pretty stable. Even though the negative charge is on a nitrogen atom, the two carbonyls serve to delocalise the charge and make this ion less reactive.
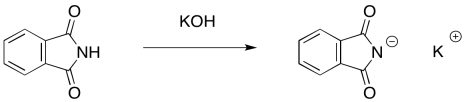
Figure NS17.11. Phthalimide is easily deprotonated.
Notice the difference in nucleophilicity between the neutral phthalimide and the phthalimide ion. In the neutral compound, the single lone pair on the nitrogen is delocalised. It is not available to act as a nucleophile. This is generally true with amides and imides; the neighbouring carbonyl ties up the nitrogen lone pair so that it is neither basic nor nucleophilic.
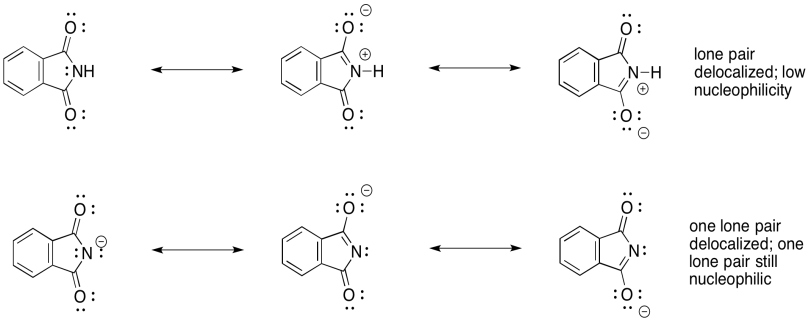
Figure NS17.12. The phthalimide anion is stabilized by resonance, but is much more nucleophilic than phthalimide.
However, the phthalimide ion has a spare lone pair. If one of the lone pairs is delocalised, then the other is still available to act as a nucleophile. Both lone pairs cannot be delocalized because they are orthogonal to each other -- that means they are forced to be in different areas of space in order to minimize electron repulsion.
The phthalimide anion is thus able to act as a nucleophile. It can donate to an alkyl halide and displace the halide anion.
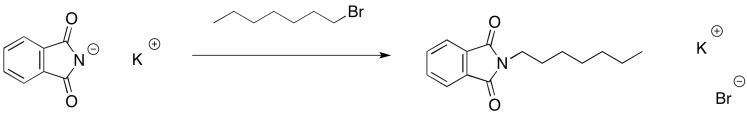
Figure NS17.13. Nucleophilic substitution with phthalimide.
The phthalate part of the molecule has now served its purpose. It was just there to deliver the nitrogen in a way that was stable, yet suitably reactive. We can get rid of it now through base-catalysed hydrolysis. Remember, that is a carboxyloid substitution reaction. The resulting phthalate salt is easily removed because of its very different solubility properties. It can be washed away with some water.
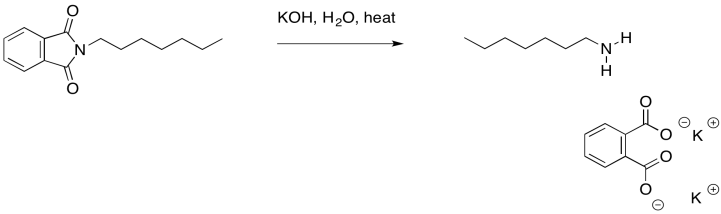
Figure NS17.14. Hydrolysis of the N-alkyl phthalimide.
Problem NS17.1.
Provide a mechanism for the hydrolysis of the imide shown above.
Problem NS17.2.
Provide products of the following reactions.
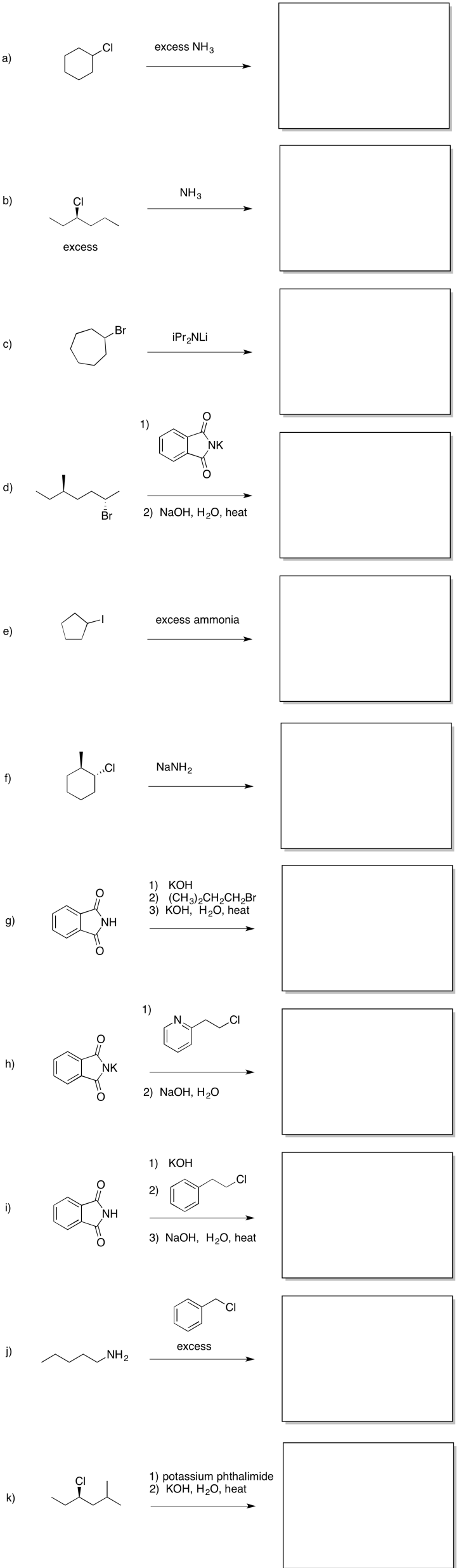
Problem NS17.3.
For the reaction of nucleophile with iodomethane in acetone, rank the nucleophiles in order of reactivity (1 = fastest, 3 = slowest).
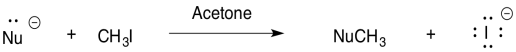
a) _ HO- _ HS- _ H2O
b) _ H2O _ H3O+ _ NH3
c) _ CH3CH2NH2 _ (CH3)2CHNH2 _ (CH3)3CNH2
Problem NS17.4.
DNA bases (adenine, guanine, cytosine, and thymidine) contain nucleophilic nitrogen atoms, which is why many halogenated compounds are carcinogenic. Alkylated DNA can still function in its process of replication, though it will do so abnormally, resulting in mutations in the DNA and, ultimately, cancerous cells.
a) Propose a mechanism, with arrows, for the alkylation of guanine:
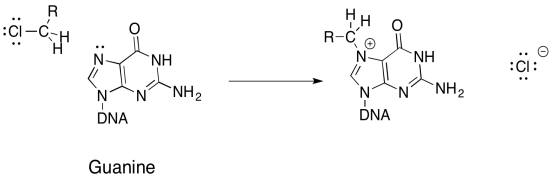
Damaging DNA via alkylation can also be used to treat cancer. The key is that cancer calls grow and divide more rapidly than normal calls, and thus are more susceptible to mechanisms that damage DNA and impair its functions. Mechlorethamine is one such drug that cross-links DNA.
The mechanism for the activation of mechlorethamine is shown below:
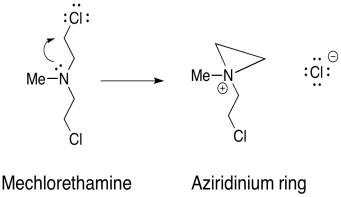
b) Using that step, provide a mechanism, with arrows, for the formation of two cross-linked guanine molecules.
c) What makes the aziridinium ring so electrophilic?
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: