Reactivity in Chemistry
Aliphatic Nucleophilic Substitution
NS13. Regiochemistry in Elimination
Sometimes, an elimination reaction could lead to formation of a double bond in more than one place. If the halide is on one carbon and there are protons that could be removed on either side, then taking one proton or the other might lead to two different products. This reaction could have different regiochemical outcomes, meaning it could happen at two different places in the molecule.
What factors might influence which product forms? We might think about product stability, in case there are corresponding differences in barriers leading to those products. We already know about stereochemical effects in alkene stability, but what about other effects?
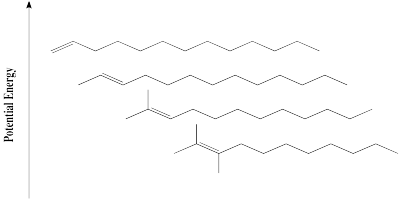
It is well-established that alkene stability is influenced by degree of substitution of the double bond. The greater the number of carbons attached to the double bond, the more stable it is.

Figure NS13.1. The trend in alkene stability.
That effect is related to hyperconjugation. Specifically, it's an interaction between bonding orbitals and antibonding orbitals on neighbouring carbons. The interaction allows the bonding electrons to drop a little lower in energy through delocalization. The interaction also pushes the antibonding orbitals a little higher in energy, but since they have no electrons, they don't contribute to the real energy of the molecule. Overall, the molecules goes down in energy.
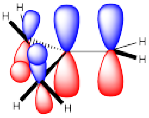
Figure NS13.2. Hyperconjugation in an alkene.
However, alkene stability isn't the only factor that plays a role in elimination. Steric hindrance can play a role, too.
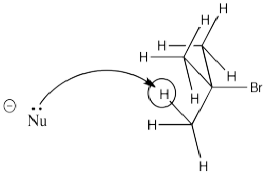
Figure NS13.3. The β-hydrogen in a bromoalkane.
In a case in which there are two different hydrogens from which to select, the one leading to the more-substituted double bond is sometimes a little bit crowded. That leaves the base with fewer viable pathways to approach the proton.
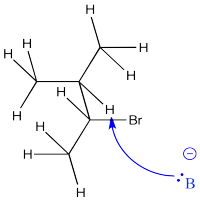
Figure NS13.4. The base approaching the more crowded β-hydrogen.
On the other hand, the removal of a proton leading to the less stable alkene is often less crowded, allowing the base to approach much more easily from a number of angles.
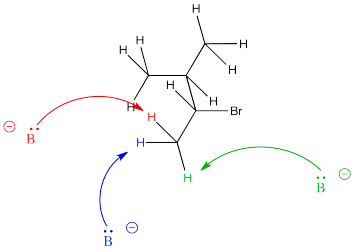
Figure NS13.5. The base approaching the less crowded β-hydrogen.
Problem NS13.1.
In the following reactions, more than one elimination product is possible. Draw the products. Circle the most stable product.
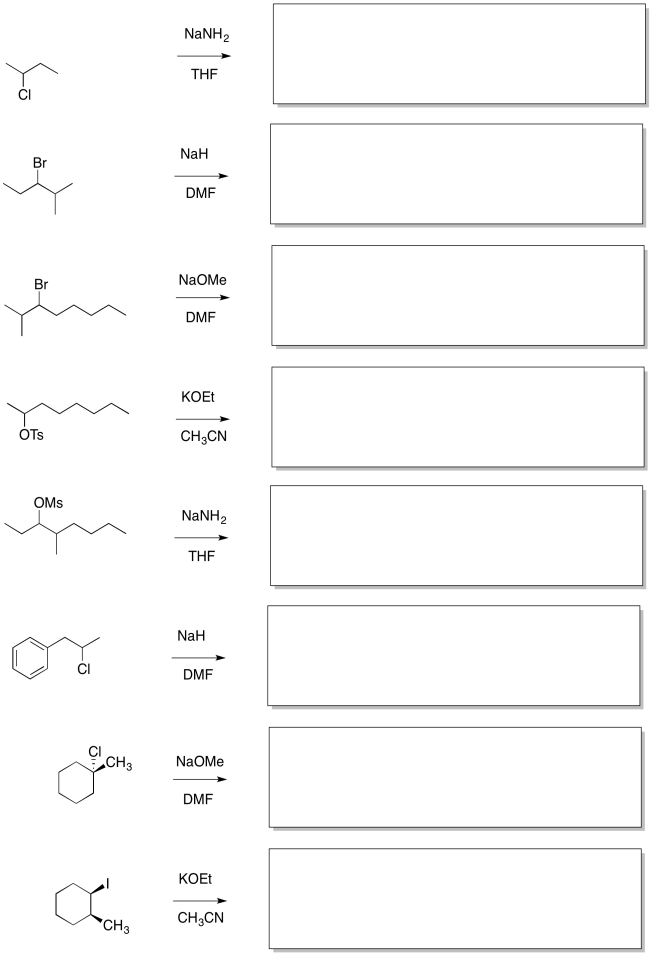
Problem NS13.2
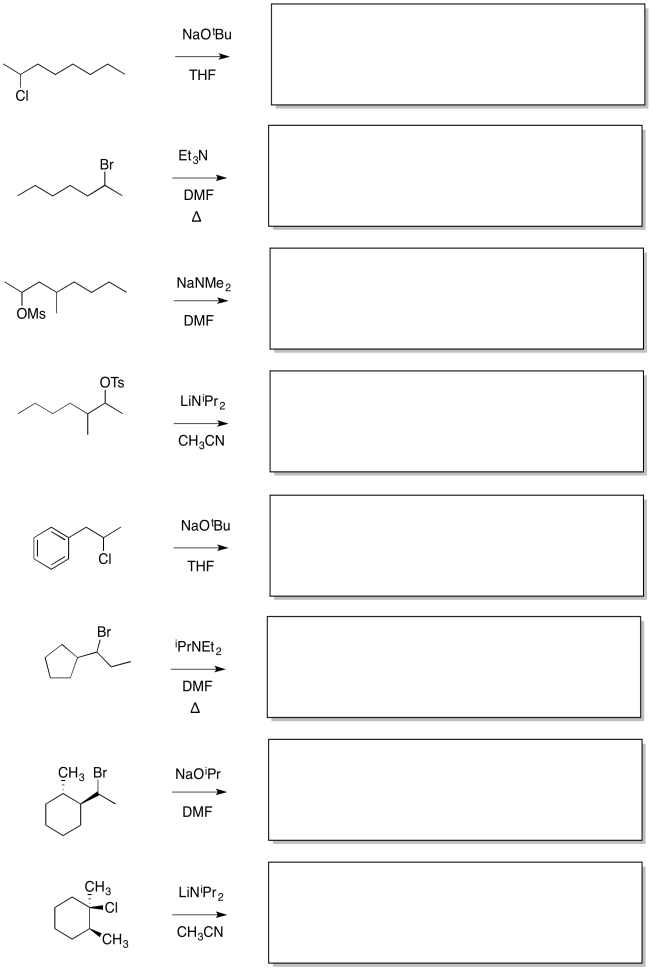
In the following reactions, more than one elimination product is possible. Draw the products. Circle the product formed via removal of the most accessible proton.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: