Reactivity in Chemistry
Aliphatic Nucleophilic Substitution
NS11. Addition to Strained Rings: Epoxides
Oxygen is a very common element in all kinds of compounds, whether they are biological molecules, minerals from the earth or petrochemicals. Exploiting oxygen's electronegativity and giving it a little help to become a leaving group is a common way to make connections and build new molecules in nature, the laboratory or the production facility.
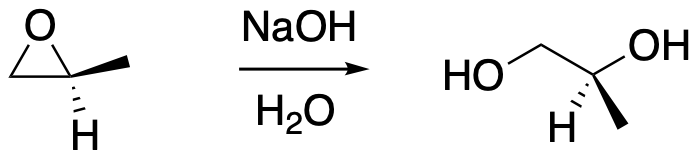
Sometimes oxygen doesn't need much help to become a leaving group. Epoxides, or oxiranes, are three-membered ring ethers. They are good electrophiles, and a C-O bond breaks easily when a nucleophile donates electrons to the carbon. For example, a strong nucleophile such as hydroxide ion is able to open these strained ethers. Usually, if the epoxide isn't symmetric, an anionic nucleophile donates to the least-substituted side where there is less steric crowding.

Figure NS11.1. Ring-opening of an epoxide or oxirane.
The mechanism of reaction is usually SN2-like. This generality is especially true with strong, anionic nucleophiles. The nucleophile donates to an electrophilic carbon of the three-membered ring, breaking the C-O sigma bond as the σ*CO orbital is occupied. The resulting alkoxide ion picks up a proton from the solvent, if the solvent is protic. Otherwise, dilute acid is added in a second step to protonate the alkoxide ion.
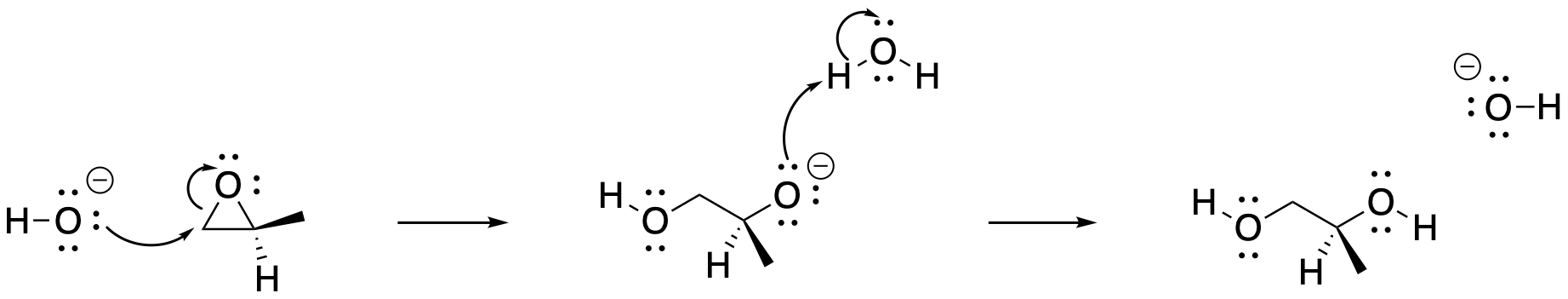
Figure NS11.2. Mechanism of epoxide ring-opening with a basic nucleophile.
A potential energy diagram can help to show why epoxides are susceptible to react with nucleophiles, whereas other ethers are not. Because epoxides are strained rings, they are at higher energy relative to other ethers. The alkoxide leaving group is relatively unstable however it is formed, but compared to that epoxide starting level, it's downhill in energy.
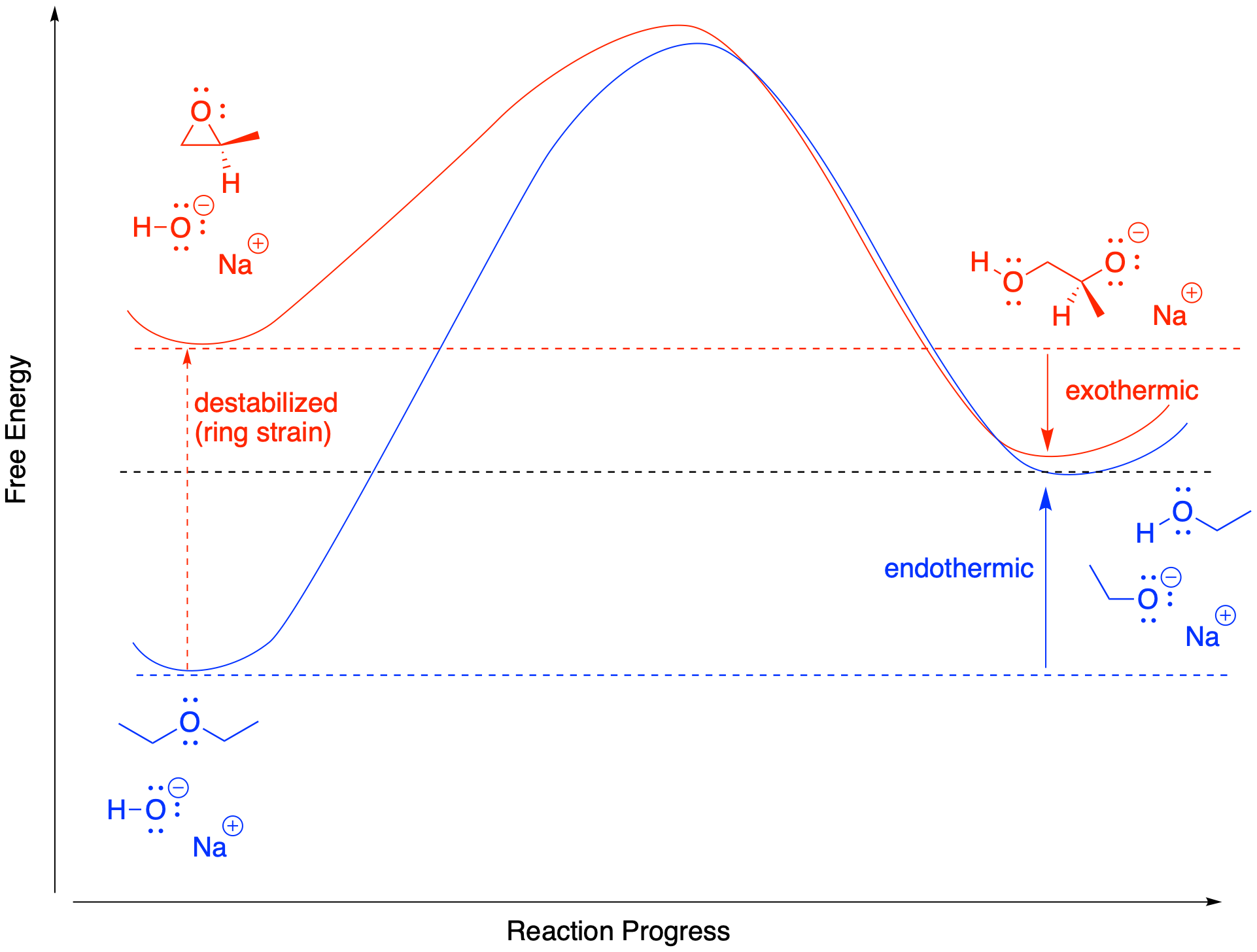
Figure NS11.3. Reaction progress diagram comparing ether cleavage in base to epoxide ring-opening in base.
Nucleophiles can add to the electrophilic carbon of an epoxide ring Nucleophilic addition leads to opening of the epoxide ring Addition of the nucleophile leads to a Nu-C-C-OH structure in the product
Problem NS11.1.
One of the most widespread uses of epoxides is in making polymers. The polyethylene glycol produced in polymerization of an epoxide is frequently used in biomedical applications. Provide a mechanism with arrows for the following polymerization of ethylene oxide.
Anionic and semi-anionic nucleophiles such as alkylmagnesium reagents are good at opening epoxides. They do so through an SN2-like pathway in which the nucleophile donates to the least-substituted carbon of the epoxide ring. Neutral nucleophiles can also add to epoxides. Sometimes neutral nucleophiles need a little help. They may require that the epoxide is activated. Activation is a step that makes something more reactive. In this case, the epoxide is activated to make it a better electrophile. As a better electrophile, it becomes more able to attract weak nucleophiles that may not be able to react with it on their own. Maybe the simplest example of electrophilic activation occurs through simple protonation. When an electrophile is protonated, it takes on a positive charge. That makes it more attractive to nucleophiles. Consequently, sometimes acidic conditions are used when epoxides are treated with weak nucleophiles. Acidic conditions can also be used simply to add halides in the form of haloacids such as HBr and HCl.

Figure NS11.4. Ring-opening of an epoxide under acidic conditions.
Under acidic conditions, the reaction proceeds via protonation of an oxygen lone pair of the epoxide. Donation of the nucleophile follows, opening the epoxide to make an alcohol with an already-formed OH group. The selectivity of epoxide opening under acidic conditions can be more complicated than under basic or anionic conditions because the mechanism shifts to being a little more SN1-like. Note that the epoxide doesn't completely open upto form a carbocation. However, there can be enough positive charge developing on one carbon of the epoxide that it becomes increasingly electrophilic. That's especially true for carbons that are more-substituted. Thus, a competition can develop between a more accessible, less-substituted carbon and a more electrophilic, more-substituted carbon, leading to mixtures of products that result when the nucleophile adds to one carbon or the other. If one of the carbons is teriary, however, a significant amount of positive charge can develop on that position. In that case, the nucleophile pretty reliably adds at the more-substituted position rather than the usual less-substituted position.
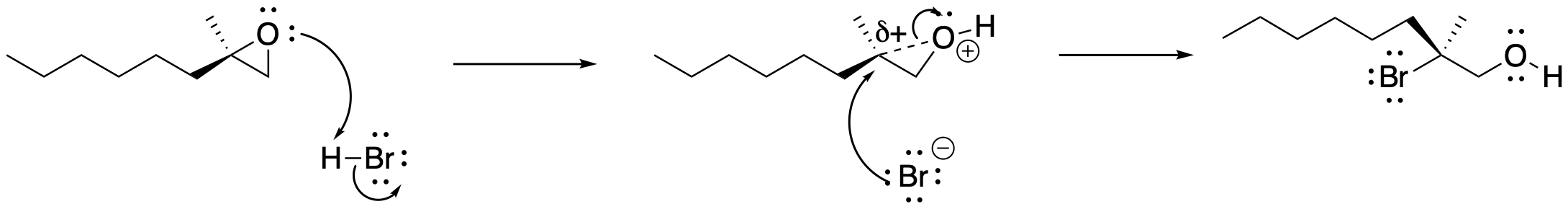
Figure NS11.5. Mechanism of epoxide ring-opening under acidic conditions.
Under anionic or basic conditions, nucleophiles add to the less-substituted carbon of the epoxide ring Under cationic or acidic conditions, competing pathways can lead to addition to either side of the epoxide ring, and sometimes mixtures of products result Under cationic or acidic conditions, if one side of the epoxide could make a very stable cation (e.g. tertiary), the nucleophile adds exclusively to that side
Problem NS11.2.
Show the products of the following epoxide ring-opening reactions.
Epoxides are very useful in the synthesis of important molecules. The Nu-C-C-O motif that is formed in nucleophilic addition to an epoxide is very valuable. Whereas other nucleophilic additions simply replace a halide or leaving group with a nucleophile, exchanging one reactive site with another, addition to an epoxide makes a product that has gone from having one reatcive site to two reactive sites. That can open the door to lots of useful strategies when trying to make a valuable commodity.
Problem NS11.3.
Show how you could carry out the following transformation. More than one step is involved.
Other three-membered rings that contain heteroatoms are also susceptible to ring-opening with nucleophiles. For example, aziridines are three-membered rings containing nitrogen. Like epoxides, aziridines can be opened with a variety of nucleophiles, such as alcohols, thiols, or other amines.
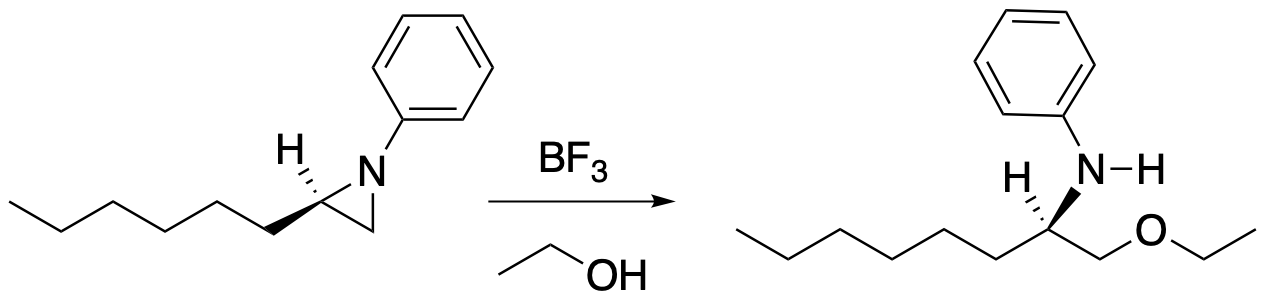
Figure NS11.6. Aziridine ring-opening.
Note that the conditions for aziridine ring-opening are different from epoxides. A nitrogen anion is a worse leaving group than an oxygen anion, so it doesn't open quite as easily as an epoxide. Aziridines tend to open under conditions that activate the aziridine by making it a little more electrophilic and making the nitrogen a better leaving group. Lewis acids, for instance, can promote the opening of aziridine rings. Because nitrogen is a good Lewis base, it binds to the Lewis acid, taking on a formal positive charge and becoming more attractive to the nucleophile. Lewis acid-base complexes tend to form reversibly, so that nitrogen can back away from the Lewis acid again after protonation.
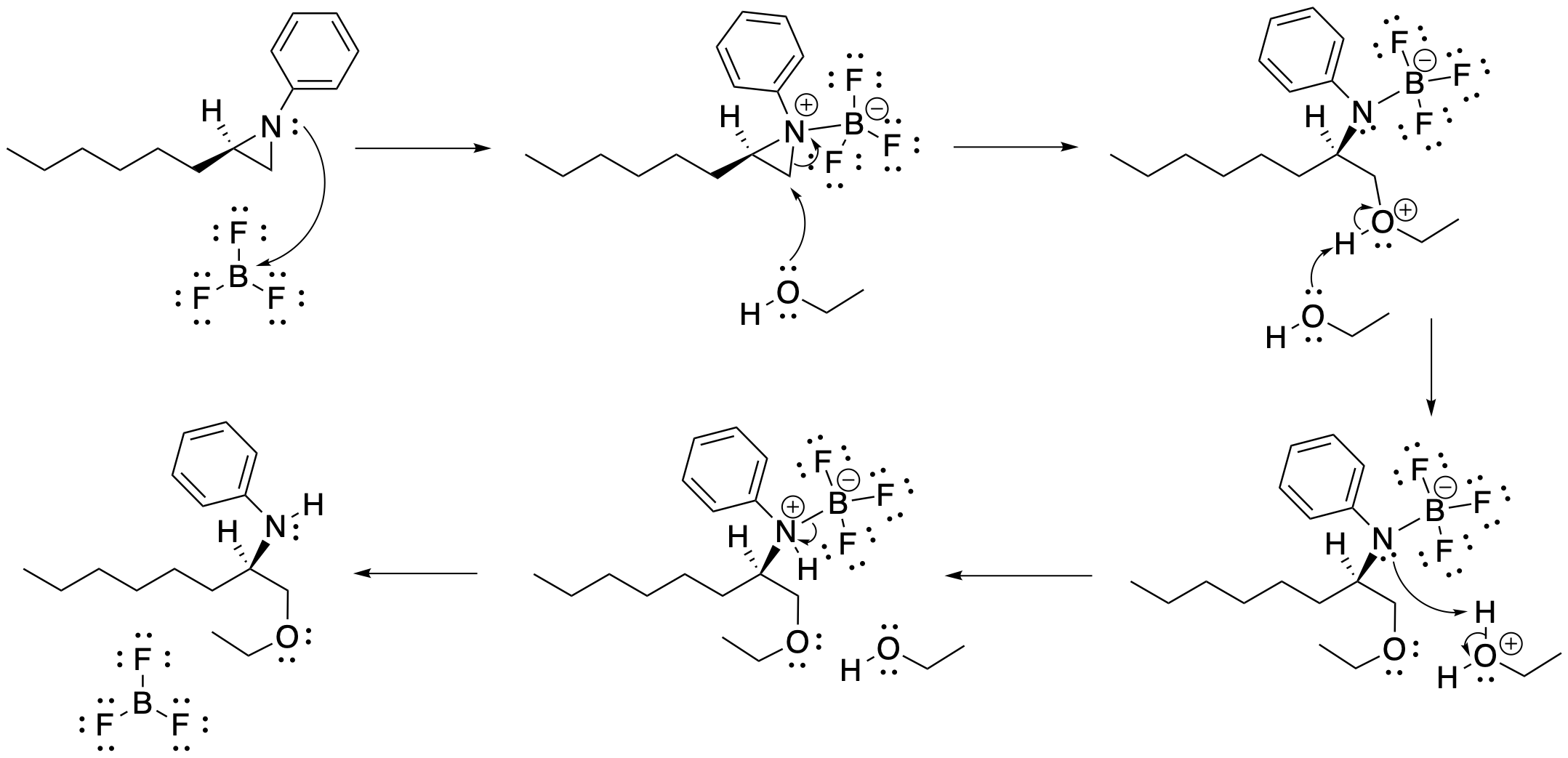
Figure NS11.7. Mechanism of aziridine ring-opening.
Despite these cationic or acidic conditions, aziridines usually undergo addition at the less-substituted carbon under the conditions shown. The exception is if one of the carbons of the ring could form a very stable cation: a tertiary carbon, or a benzylic or allylic carbon. In those cases, the nucleophile adds to the more-substituted position. However, it should be noted that the regiochemistry of aziridine openings depend not only on the aziridine carbons, but also on the group attached to the nitrogen, and even the nature of the activating species, whether it is a Lewis acid, a protic acid, or other activators such as acid chlorides.
Problem NS11.4.
Show the products of the following aziridine ring-opening reactions.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: