Reactivity in Chemistry
Electrophilic Rearrangement
ER1. Introduction
In organic chemistry, a rearrangement is an intramolecular process in which an atom or group of atoms shifts to another position in a molecule. Sometimes, this process leads to formation of an isomer. Sometimes, the rearrangement is accompanied by the loss of part of the molecule or the addition of another molecules, so that the product is not an isomer of the compound without rearrangement.
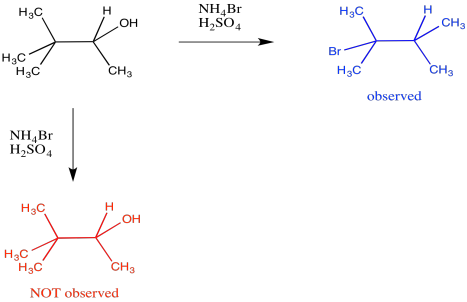
Rearrangements are often observed under acidic conditions. They often occur as complications during other reactions such as nucleophilic substitution via an SN1 mechanism. Organic cations frequently rearrange if doing so can product a more stable cation. For example, the treatment of 3,3-dimethylbutan-2-ol with bromide ion under strongly acidic conditions might be expected to result in a straightforward replacement of the OH leaving group with the Br nucleophile. That would make 3-bromo-2,2-dimethylbutane, but that isn't what is observed. Instead, the reaction leads to 2-bromo-2,3-dimethylbutane.

Figure ER1.1. A rearrangement during nucleophilic substitution to give an isomer of the expected product.
In that product, one of the methyl groups has changed positions. It has shifted from one carbon to the next. This event is called a 1,2-methide shift. When a carbocation forms, a methyl group is able to hop one carbon over to fill the hole on the cationic carbon. It takes the pair of electrons from the C-C σ-bond with it. That leaves another cation behind, but in this case, the new cation is a tertiary carbocation. It is more stable than the original secondary carbocation.
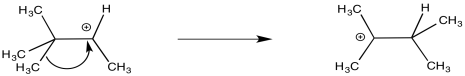
Figure ER1.2. The mechanism of the rearrangment reveals a 1,2-methide shift.
Similar 1,2-shifts also occur with hydrides (a hydrogen atom with the electron pair from the H-C σ-bond) and aryl groups (entire benzene groups). They always occur from one carbon to the carbon next to it. Sometimes, however, a series of these shifts happening one after another can make it look like a group moved much farther than that.
In this chapter, we will see a number of reactions that are loosely related to the idea of these 1,2-shifts involving carbocations. In most of these cases, an actual carbocation never forms. Instead, a group shifts over as soon as there is enough buildup of partial positive charge to draw the group in. However, if you look closely, you will find that same pattern of a 1,2-shift somewhere in the mechanisms of each of these electrophilic rearrangements.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: