02/08/2008
WHAT MAKES UP MATTER? HOW DO WE KNOW?
Chemistry is the science of matter, energy, and their interrelationships. All humans must have speculated as to the nature of matter and what constitutes it. Take for example a small copper wire, which is made of the pure element, copper. How could you show what makes it up? Several ideas can be suggested. The copper wire could be melted but you still have copper when you are done. This is an example of a physical change. You are very familiar with physical changes associated with converting water from solid to liquid to gas and back again.

You could combine it with something like water or air. It turns out copper is pretty stable in water (think of all the copper pipes we have in our houses). Copper can react with other substances to produce new substances whose properties don't resemble that of a copper wire. Such alterations are examples of chemical changes. Neither of these has led easily in a direction that would answer our question - of what is copper made? One last possibly would be to break the wire in half, then break the half in half, and continue that in our mind past the point we could actually see the copper fragments that result. At that point we keep cutting with imaginary microscopic scissors. The question is now: how many cuts can we make. Is there a point when we can't cut the copper particle in half and still have copper? Or can even the tiniest particle of copper be cut infinitely and still result in copper particles (much like a number line between the numbers 1 and 2 can be divided an infinite number of time. This question puzzled the ancient Greeks as well. Was matter continuous and hence cut be "cut" an infinite number of times, or is it discrete. Democritus (450 BC) believed that matter consisted of tiny small and indivisible particles called "atoms".
THOUGHT EXPERIMENT: CUTTING A COPPER WIRE IN HALF....
Answer to the thought experiment.
His ideas where not derived from experimentation, but rather by intuition and philosophical thought. It turns out he was almost right. Contemporaries expanded on this concept and believed that the world consisted of 4 elements, earth, water, air, and fire. We now recognize that matter is made of atoms. It turns out their are 92 different types of atoms that occur naturally in our world. Each differs from the others in their properties (such as size, reactivity, etc.). However, we now know that atoms are divisible, and consist of a nucleus with positively charged protons, neutral neutrons, and negatively charged electrons moving around the nucleus. The 92 naturally-occurring atoms differ from each other in the number of protons and electrons they have. The properties of different atoms are displayed in the periodic table. The atomic number on the periodic table representation of a given atoms gives the number of protons and electrons in the neutral atom of these elements.
Now consider the same experiment only with solid water. Melting or reacting the water chemically confuses our understanding at this point. If, however, we do the same thought experiment and cut a cube of ice in two and continue as above, we reach a point that we have two particles of water which can not be cut again and still be water. Do atoms of water remain, as we had atoms of copper? A quick view of the periodic table shows that there are no atoms or elements called water. From your high school study of biology and chemistry, you remember that water is symbolized as H2O. The smallest particle of water consists of 2 atoms of the element H attached through chemical bonds to an atom of the element oxygen. The resulting particle is called a molecule - 2 or mores atoms bonded to each other to form a stable species. These bonds must be strong since they are not broken when solid water melts or liquid water evaporates. Water, whether it is solid, liquid, or gas, is still H2O. As we will see later, the bonds (which we represent as lines connecting the atoms) represent negatively charged electrons which are attracted to both atom's' nuclei. These bonds can be broken in a chemical reaction in which water is changed into derivatives (H, OH, H, or O) with different properties than water.
Atoms of the 92 different elements can bond to themselves and each other to form the myriad of molecules that make up our world. Using molecular models, we can make some simple molecules containing carbon (C), nitrogen (N), oxygen (O), and hydrogen (H). Remember these models are just models. Bonds are not really "little sticks" that connect atoms. There are many ways to represent molecules. The graphic below shows different representations of
METHANE (CH4), AMMONIA (NH3) AND WATER (H2O)
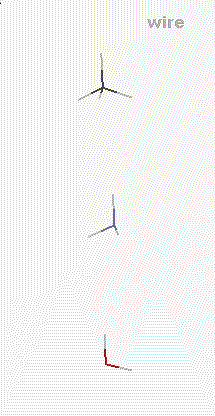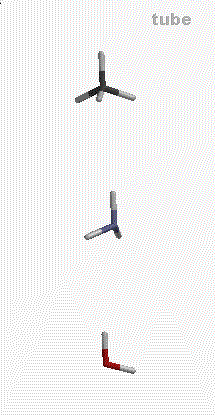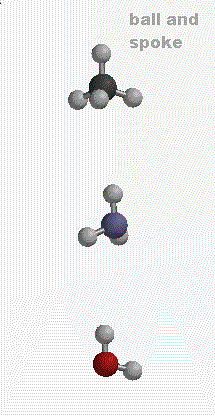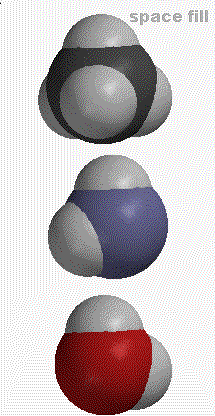
Definitions (taken from Chemical Principles by Atkins and Jones):
Molecular Modeling on the Web: Using Chime and Jmol
If you are using a computer in one of the access labs on campus, you should use your browser to see and manipulate with your mouse different representations of molecules like water. Programs called Chime or Jmol allow you do to that.
To visualize molecules using Chime, you must either be in a public PC lab to view the structures or download Chime (its free) to your own PC. To do so, go to the Symyx/MDL Web Site link for Chime. You will be prompted to register with a usrname and password, before you get the free download. Jmol structures can be viewed on any computer without downloading anything.
I will place an animated DNA double helix structure for Chime or Jmol files adjacent to the link. Click on Water below to view the image with Chime. A image will appear in the right hand window. To get back, select the Back button on your web browser.
Explore the possibilities. For more information on commands, go to Chime Instructions.
Now select the Jmol link above to view water.
We will be studying ethanol, the alcohol found in beer, wine, and distilled sources. Select ethanol below and the various buttons in the left frame to change the rendering of ethanol.
MIXTURES:
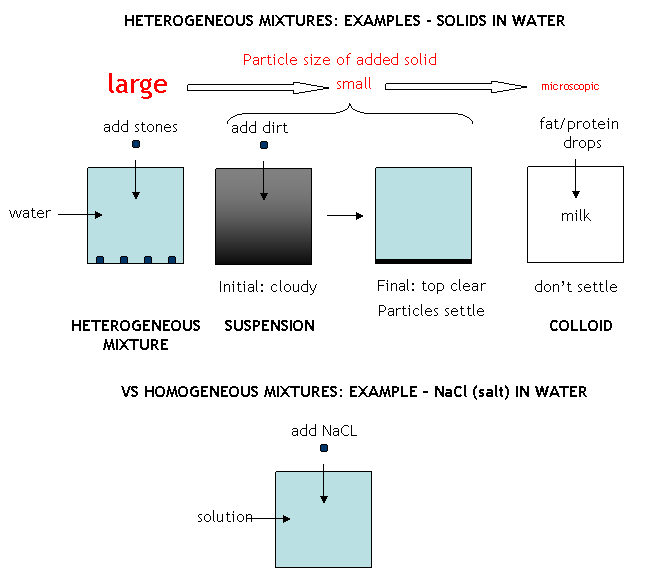
We discussed that matter usually never exists in a pure state in nature. Rather, pure substances are combined to form mixtures. Mixtures can be separated into heterogeneous and homogeneous mixtures. Consider mixtures composed of a solid added to a liquid like water. In a heterogeneous mixtures, the solid particles can be readily discerned in the liquid, such as the case of sand or rocks (particles of relatively large size) in water. The solids in this case can be separated from water filtration. In some cases, the solid particles are of intermediate size, and soon settle out after addition to water. Such a heterogeneous mixture (for example, dirt in water)is called a suspension. The dirt particles can be separated from the water by filtration. If the particles are microscopic and can't be seen with the naked eye (such as in milk or blood), the particles stay suspended and don't settle out. This kind of mixture is called a colloid. The microscopic particles in a colloid can be separated from water by high speed centrifugation or with some specially designed filters. An unsettle suspension and colloids are cloudy, and light passing through the mixture scatters from the particles.. In homogenous mixtures, the particles are so small that they never separate on standing or in simple centrifugation, and do not interfere with light passing through the mixture. Hence the mixture appears clear. Homogeneous mixtures are also called solutions. Mixtures can be separated into component parts by purely physical process, including evaporation, condensation, filtration, sublimation, deposition, etc. The components of the mixture must have different physical properties which allow the components to be separated from each other.
mixture: a type of matter that consists of more than one substance and can be separated into its components by making use of the different physical properties of the substances present.
HISTORY OF OUR UNDERSTANDING OF MATTER AND ITS TRANSFORMATION - SELF STUDY IN CRITICAL THINKING
Hopefully you have had a chance to read the syllabus. One of the goals of this class is to "develop critical thinking skills that you can transfer to your other courses, your profession, and your daily life". Just what are the characteristics of critical thinkers? The following comes from a book, Challenging Your Conceptions, by Randolf Smith. According to him, critical thinkers:
When faced with an issue or problem, critical thinkers will develop as many possible answers, solutions, or explanations for the issue or problem that they can. Then they devise ways to differentiate among the proposed solutions.
Things often are not what they appear to be. Matter appears solid in nature, but we know that overall size of the atom consists mostly of the volume occupied by the electrons around the atom's nucleus, which contains almost all of the mass of the atom. How do scientist go about developing their ideas - laws, hypotheses, theories? Does our intuition about the world lead us to the right ideas about how the world works and what is is made of?
Consider this simple example, which I will explain in class: A string around an orange and the earth.
The early Greeks thought the matter consisted of four elements, earth, water, air, and fire, as we discussed in class. As time passed, ancient Greek thought was supplemented with experience and experimentation. The protoscience of alchemy led to the synthesis of new types of substances but also to great confusion in the theories used to explain how matter was transformed. The use of measurement greatly assisted the attempts to make progress, but misinterpretation of data often obscured the path to truth. You have already confronted one such experiment in your group critical thinking questions.
Jan Baptista van Helmont did the following experiment which was published posthumously in 1648. He took a 5 pound willow tree growing in 200 lb. of soil and weighed the tree alone after 5 years during which it only received water. The tree grew to 169 lb. but the soil weighed just a few ounces less than 200 lb.
From where did the 164 lb. of additional tree weight come? Discuss in small groups.
Perhaps the biggest barrier to advancing our understanding of matter came from the wide-spread acceptance of pholgiston theory. Any matter that can burn was thought to contain pholgiston, which escaped into the air on burning. This suggested that burning would decrease the weight of the object, which it apparently does if you consider the burning of a log. Only ash apparently results, but of course early people didn't think about the mass of the escaping gases (carbon dioxide and water). However, when metals were burned the weight increased. Supporters of the theory said that pholgiston had negative weight! The air was required for burning so it was assumed that the air was low in pholgiston. During burning, pholgiston flowed from the burning substance to the air like water flows down a stream.. Priestly found that if an red mercury compound (which we now know as an oxide of mercury) was heated, a gas formed, which would vigorously support combustion and the respiration of a mouse. He had purified oxygen but was so steeped in pholgiston theory that he thought the substance to be dephologisticated air. To the adherents of phologiston theory, for combustion to occur, something (phlogiston) must be depleted or absent form the air, so that the pholgiston from the burning object could enter it.
Lavoiser, the father of modern chemistry, settled the problem (in the 1780's). He formed the mercury oxide from mercury and air and found the weight of the air decreased by 1/5 to 1/6. When he heated the oxide he formed a gas which had the same weight as the amount of gas removed from the air in the first place. Hence this gas, which he named oxygen, is necessary for combustion. He revolutionized our understanding of chemistry and matter. In addition he came up with the Law of Conservation of Matter - In a chemical reaction, matter is not created and destroyed, only interconverted. When you burn a log in air, the mass of the log + the mass of the oxygen combined with the log = the mass of the ashes and gases produced, which people can't see.
Dalton, in the early 1800's developed the Atomic Theory, using Lavoiser's work on mass conservation and Proust Law of Constant Constant Composition. This law is easier than it sounds. Consider a cake as an example. You distribute to all your friends a recipe for a chocolate cake. If everyone follows the recipe, all cakes could have the same number of grams of chocolate in a given amount (let's say 100 grams) of cake. The same with pure substances. If you break the chemical bonds which hold hydrogen and oxygen together in 100 g of water, you always get 11.2 g of hydrogen and 88.8 g of oxygen from the 100 g of water. That is, water consists of 11.2% by weight of hydrogen and 88.8% by weight of oxygen, no matter where the water comes from. Nature always follows the same recipe for water - H2O.
Dalton used these ideas and his own experiments to come up with a way to explain all these ideas - which ultimately became known as the atomic theory.
Scientific Laws, Hypotheses and Theories: Is there a scientific method?
Our first lab will be on the "scientific method". You have probably studied this ever since you were in Middle School, and might feel you have a reasonably good understanding of it. However, many people now suggest that there is no "scientific method" that all scientist follow to discover truths. A clear conception of scientific laws, hypotheses and theories might help in understanding how new ideas arise. Let's consider a specific example, with which you have some familiarity.
Imagine a gas in a cylinder that has a moveable piston. The piston can go up and down. Now let's do a series of experiments in which we change one parameter or variable and measure the effect on another.
1. Increase the temperature (T) of the gas in the cylinder and measure the volume (V) of the cylinder. Let all these experiments be kept at constant external pressure (the atmosphere) to ensure that pressure (P) changes won't cloud our understanding of the results. The piston can move freely to keep the pressure inside the cylinder the same as the outside pressure. In this case the temperature, T, (what we vary) is the independent variable. What changes in response to the T changes is the volume, V, which is called the dependent variable (its value depends on the value of the independent variable). I bet you can guess that as the T increases, so does the V of the cylinder. (i.e. the piston moves up). If you did this experiment enough with different gases, you would always get the same relationship: the V increases in a linear fashion with increasing T. In mathematical terms we say that V is directly proportional to T (V α T). This mathematical equation for describing this relationship is:
V = kT
where k is a constant. This simple equation summarizes lots of experimental data. We now call it a scientific law, a succinct statement that summarizes a lot of data but which does not explain "why" or give a reason to explain this phenomena. This equation is a mathematical law.
2. Now do a similar experiment but vary the external P on the cylinder (the independent variable) and determine the effect on the V of that cylinder at constant P. Again you can probably guess the results: as P increases, V decreases. In mathematical terms it turns out that V is inversely proportional to the P, or V α 1/P. In an equation form, another law arises:
V = k/P or PV = k.
3. In a final experiment, we can vary the amount of gas (n) in the cylinder at constant P and T, and see the effect on the V. Again you could imagine that V α n. In mathematical form, another law emerges,
V= kn
You can visualize these experiments by using an "applet" at the link below. When you select the link, select "applet" on the new page. A series of new windows will appear that will allow you to change T or P while holding the other variables constant. Run two different simulations:
1. Force = ideal; T = constant. Let x = V, varying it from 900 to 100; Let Y = P. A better simulation here would be to let the x variable be P (what you actually alter in the lab) and the Y variable V (see how V depends on P) but the simulation does not allow that.
2. Force = ideal, P = constant. Let x = T. varying from 200 to 400; Let y = V.
Now we mathematically combine all 3 of these laws to get:
V = nRT/P OR PV =nRT where R is a constant.
This is called the Ideal Gas Law. This, as well as the other three laws, are empirical laws, derived from experimentation. They can be used to predict the value of V, n, T, or P if at least three of them are known. The ideal gas law does not explain "why" this law hold, or the reasons for these interrelationships. We now need something more. We need an educated guess (an hypothesis) that might explain the laws.
One such hypothesis would be that gas are made up of particles (such as atoms or molecules) moving around at high speeds. They bang into the walls of the cylinder wall and piston bottom, causing pressure. The higher the T, the faster they move, which would lead to higher P inside the cylinder causing the piston to move up, expanding the volume. Such an hypothesis can suggest news experiments, whose results could be predicted by the hypothesis. The predictions can be deduced from the hypothesis (hypothetico-deductive reasoning). Eventually the weight of years of experiments that produce only results that support the hypothesis give us reason to believe that the hypothesis, now transformed into an explanatory theory, works so well since it is true. The theory used to "explain" the gas laws, and which can be used to actually derive the ideal gas law, is called the kinetic molecular theory. The word theory is only given to explanatory ideas and hypotheses that have stood the test of time and arduous experiment. To question a theory by saying that it is just a theory is a worthless and trivial argument.
Theories, however, are not ingrained in stone. Some theories that have been devised and seem to have explanatory power have fallen by the wayside. Newton, one of the greatest scientific geniuses of all time, came up with an incorrect theory to explain the property of gases. He thought gases consisted of immovable particles (not particles zipping around at high speed), which behaved as springs (i.e. the particles were held apart by repulsive forces which mimicked springs. As the external pressure on the piston increases, the volume decreases as the springs come together. He was able to derive the PV=k law with his wrong theory. Limitations in his ideas soon became apparent as gas molecules were found to be moving at a very high speed.
Old theories become subsumed under new theories with more explanatory power. These new theories are derived to explain anomalies in results that can not be explained by the existing theories. For example, Einstein's Theory of General Relativity offered a new way to understand gravity in situations that Newton's laws of gravity could not explain. However, we still use and study Newton's Laws which help us land spacecrafts on asteroids and thread them through the rings of Saturn.
There is another extremely important feature of scientific theories that differentiate them from nonscientific explanations of the world based on philosophy or theology: scientific theories must be falsifiable. That is, they can be shown to be wrong. If a theory can't be falsified, then it is not a scientific theory. A classic modern example is intelligent design. No experiment could ever be performed to disprove that "theory". Hence it is not a scientific theory and should not be taught in a science class. In truth, scientific theories can never be proven, since all possible experiments that might lead to a finding inconsistent with the theory can't be done. However, one instance of a result which is not in accordance with a theory can bring a theory down.
The main champion of the idea that scientific theories must be falsifiable was Karl Popper. He suggested that the best scientific theories are those that would predict the most unexpected phenomena. An example is Einstein's explanation of gravity. Newton's Theory of Gravitation explained gravity as a force which propagates between space between two massive bodies. The force of attraction (there is no repulsion in gravity) is inversely proportional to the square of the distance between the centers of mass of the two objects. A question that arises that Newton could not explain is how such a force would propagate through a vacuum. Einstein suggested that gravity is the result of the bending of space by a large object. To visualize this, think of a taught rubber sheet. Place a large metal sphere in the center of the sheet. The sphere bends the 2D sheet around the ball. Now roll a smaller metal ball on the stretched sheet. If it is not going to fast, it will bend toward the large metal ball and might even hit it. These balls are two small for gravity between the two balls to force them together. Rather the small sphere follows the contours of space toward the large sphere.
Now consider a massive object like the sun. In analogy to the 2D rubber sheet example, it too would bend space-time around it. Even light passing by the sun would follow the contours of the bent space. This theory might sound outlandish, but it is exactly the kind of theory Popper would say is among the best. It can be tested by determining if light bends (as it follows the contours of space) as it passes by a large object. A test of this idea was performed in 1915 during a total eclipse of the sun. The results were in accordance with Einstein's Theory of Relativity, and has since been reconfirmed many times with instrumentation unavailable in Einstein's time. The link below shows a graphic illustrating the experiment, which will be explained in class.
Einstein, Gravity, and an Eclipse of the Sun
An earth-sized planet discovered: Gravitational lenses
In setting up a typical experiment, one variable, the independent variable, is changed, and the effect of the change is observed on another variable, the dependent variable. Other potential variables that could affect the dependent variable (such as T, P, V, or n in the gas labs above) are controlled by keeping them constant. Sometimes a correlation is observed between the independent and dependent variables. For example, we saw that P, the dependent variable, was a linear function of T. Inverse correlations are also observed. The extent of correlation can be determined statistically by calculating a correlation coefficient. The graph below illustrates correlation graphs.
Just because a correlation is observed does not mean that that changes in the independent variable causes the change in the dependent variable. Perhaps a third, unknown variable, the co-varies with the tested independent variable, actually causes the change in the dependent variable. Causation is more likely in a correlative study when rigorous attempts have been made to exclude (by appropriate controls and analysis) confounding variables and biases.
Many studies (particularly medical ones) in the popular press purport to show how a particular diet, for example, causes a medical problem, instead of indicating that it is just correlative. For an assignment, please read the link below which deals with these issues.
Reading: Correlation and Causation
The above description of how science moves from observation to laws to hypotheses to the theories should not suggest that the process in linear. Theories, when tested, beget new observations which could either support the theory or lead to its falsification. The process is more cyclical. Even then, it is not followed in such an orchestrated fashion. Sudden flashes of insight, often through analogies with ideas from nonscientific ideas, or even dreams, led to new ideas and ultimately new ideas. Science is after all a human endeavor.
Modern Concept of the Atom:
Dalton's Atomic Theory has stood the test of time, with one major change. Modern evidence shows that atoms are divisible and are composed of protons (+1 charge, 1 atomic mass unit - amu) , neutrons (neutral, 1 amu), and electrons (-1 charge, about 0 amu). Obviously 1 amu must be a very small weight - much smaller than a pound or a gram. It has been shown that 1 amu = 1.67 x 10-24 grams. Electrons have a tiny mass but it is 1/2000 that of a proton or neutron, so we'll just round its mass to 0 AMU. The total mass of an atoms is contributed by the protons and neutrons, with little contribution from the electrons. The mass number is simply the total number of protons and neutrons in the atom. Protons and neutrons reside in a very small central region of the atom called the nucleus, which makes up most of the weight of the atoms. Electrons are moving around the nucleus and are held to the atoms by the electromagnetic force. With a scanning tunneling electron microscope, we can actually now visualize individual atoms. We can actual move them as well. Check out the interesting links below:
The atomic number defines the number of protons in the nucleus and hence the number of electrons in the neutral atom. The elements of a given atoms are not exactly identical. Each neutral atoms of an element have the same number of protons and electrons, buy differ potentially in the number of neutrons in the nucleus. Different isotopes of an element have the same number of protons but different number of neutrons. The atomic weight of the different isotopes thus varies. The atomic weight shown in the periodic table is a weighted average atomic weight of the different naturally occurring isotopes, and is therefore not a whole number, as you expect if you just added the number of protons and neutrons, with each having a weight of 1. We discussed an example of chlorine, which has an atomic weight of about 35.5. Since it has 17 protons, we can hypothesize that there are two naturally occurring isotopes, one with 18 neutrons and one with 19. Each isotope would be equally abundant. (Actually there are two isotopes of Cl, one with 18 neutrons and one with 20. The one with 18 neutrons represents about 75% of naturally occurring chlorine.)
Although I mentioned that nature was simple - there are 92 naturally occurring elements, they still have markedly different properties. They can be solid, liquid, or gas at room temperature, have different densities, and clearly have different chemical properties. For example helium is pretty unreactive chemically (that's why you can breath it with no problem), but elemental sodium reacts violently with water. Can we make some simplified order out of these disparate atoms and properties? Similar endless diversity and complexity is seen in the biological world, but Linneaus came up with a taxonomy classification for the living world, based on the properties, function, appearance of organisms. Clearly chimps and humans are more closely related than cauliflower and humans. The same can be done with the 92 elements. In the 1860's (before our knowledge of protons, neutrons, and electrons), Mendeleev discovered that if he arranged the elements in a row in order of increasing atomic number, he found that at repeating intervals, the next element in the row seemed to have properties very different from the preceding element (i.e. a solid metal instead of a gas) but similar to an earlier element in his organization. He interrupted the extension of the row and started a new row with the new element directly underneath a previous element with similar properties. He didn't know all the elements that we know today, but he was intuitive enough to leave gaps in his rows to optimized the alignment of the elements into columns with similar properties. His achievement was remarkable and is the basis of the modern periodic table. Elements in the table are arranged in way to shown how the chemical and physical properties of elements repeat in a periodic fashion. The periodic table really represents a nonmathematical law, which allows the prediction of the chemical and physical properties of elements but does not explain the origin of those properties.
Arrangement of Electrons:
If all the atoms of different atoms looks about the same - fuzzy balls of moving electrons zipping around a small nucleus, how could you account for the diversity in chemical and physical properties of atoms? How can you account for the arrangement of atoms in the periodic table? Modern chemical theory and experiments shows that electrons are not moving randomly around the nucleus but rather exists in "shells". The first period of the periodic table has one shell that can hold 2 electrons. The second period has two shells, an inner shell with 2 electrons, and an outer shell with up to 8. The third has three shells, etc. More sophisticated theory and analyses shows that the electrons are arranged in subshells within the shells. Subshells consist of orbitals named s, p, d, and f. and each orbital can hold two electrons.
Quiz: Atoms and Molecules
We call this arrangement of electrons the electronic configuration of the atom. As we proceed left to right in a period (row) of the periodic table, we add one more electron to the atom. Electrons are added to the lowest energy level orbitals available as we build up the electronic configuration of atoms. Electrons are added one after another to the orbitals in order of lowest to highest energy (see link below), with no more than two electrons in any orbital. Eventually the shell is filled (for elements in Group 8 - the Noble Gases) and in the next element (for elements in Group 1A) , the electron is added to a new outer shell.
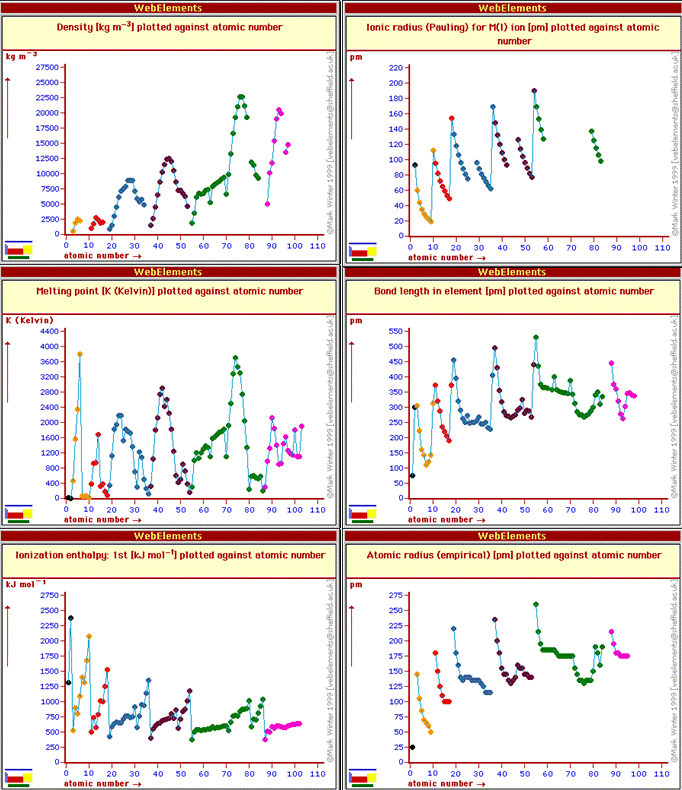
The periodic table is really a graphical representation of periodic law: that the chemical and physical properties of elements are periodic functions of the atomic number. We will (have) discuss(ed) in class how the ionization energy increases across period 1 (from H to He), and then drops precipitously for Li in the start of period 2, followed by a general increase across period 2 (from Li to Ne) before another precipitous drop for Na, the start of period 3 elements. The drop at the start of each period arises because it is easier to remove an electron from an atoms the farther away the electron is from the nucleus. At the start of each period the added electron is in a new shell further from the nucleus than the inner shells. Other periodic trends are shown in the figure below which was made from the Web Elements periodic table web site.