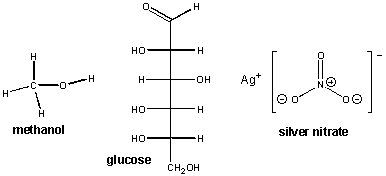
Acid/Bases
Breaking Bonds: Charge transfer reactions - Acid/Base and Oxidation/Reduction (Redox) Reactions
Chemical reactions involve making and breaking bonds. So far we have considered making and breaking ionic bonds between ions in aqueous salt solutions. Now we want to discuss making and breaking covalent bonds. When we break a covalent bonds, there are three ways we can separate the two atoms involved in the bond. Consider for example a C-X bond. If when the bond is broken, the electrons are left with C, C takes on a negative charge and is called a carbanion. If the electrons leave with X, then C is left with a positive charge and is a carbocation. If one electron stays with each atoms, the C has no charge, but does not have an octet. The carbon is called a free radical.
When the two electrons in a bond between X and H remain with X, a proton (H without its electron) is cleaved from the molecule. An isolated proton is very reactive and will bind to another molecule which can accept it. A reaction in which a proton is donated to another molecule is called an acid/base reaction. If electrons are transferred from one substance to another, the reaction is called a oxidation/reduction (redox reaction). We will first consider acid/base reactions.
Acid/Base Reactions
First, let's backtrack and consider what types of substances, when dissolved in water, allow for the conductance of an electric current. Remember, for the light to light, ions must be present in solution. Previously, we saw that an aqueous NaCl solution conducted electricity. Obviously the ionic bonds holding Na and Cl must be broken when solid NaCl dissolves in water which results in separate Na+ and Cl- ions. What about MgCl2? Of course it must since it is a salt, held together with ionic bonds, that dissolves in water and forms ions. What about AgNO3? Likewise this should as well since it is also a salt of the metal Ag and the polyatomic ion NO3- which separate into individual Ag+ and NO3-ions. (Remember the N and O atoms in this polyatomic or molecular ion, which are held together by covalent bonds, stay together). Now let's consider compounds not held together by ionic bonds. Consider for example, methanol, [CH3OH(l)] and glucose [C6H12O6(s)]. There structures are shown below:
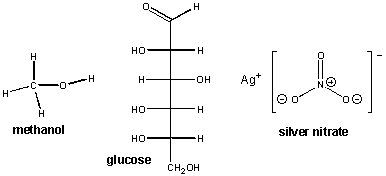
When these substances were dissolved in water, no light was observed. This implies that were no ions in solution. From these examples, we can tentatively conclude that covalently bonded species don't dissolve in water to form ions. This makes sense in that pure H2O itself, a molecule held together by polar covalent bonds, did not conduct electricity in our experiment.
Now consider one last example: HCl(aq), made by dissolving HCl(g) in water. In this demonstration, a bright light was observed. The only way we could get a light in this solution is if charged ions were present. Yet HCl is a neutral molecule. How could such charged ions be produced. The best possibility is that the H-Cl bond was broken and the electrons went to Cl making it Cl- with 8 outer shell electrons. We saw a similar situation when salts dissolved and the ions that comprised the salt dissociated (or separated from each other) to form individual ions. The case of HCl is different. It is a covalently bonded molecule - i.e. it is not made up of ions. However, when the HCl bond breaks, ions are formed. We call this process ionization (compared to dissociation of ionically bonded salts). The H would be left with no electrons and becomes H+ which is simple an isolated proton. Classes of molecules like HCl that are bonded covalently yet seem to give up a proton in water when dissolved are called acids. Earlier definitions of acids were that they formed H+ in solutions.
We now know that the the bond between H and Cl is not just severed by itself. Another species in solution - in this case water - attacks the HCl and pulls off the proton. The HCl donates a proton, our definition of an acid, and the water accepts a proton. A proton acceptor is called a base. This concept of acids and bases was developed by Bronsted.
The acid/base reaction discussed above can be summarized by the equation:
HCl(aq) + H2O(l) ---> H3O+(aq) + Cl-(aq).
Again, H+ ions aren't just formed in solution. Rather an agent, water in this case, pulls the H ion off to form H3O+ . Just as in redox reactions, electrons are not simply lost when a substance gets oxidized, but rather are "pulled off" by a oxidizing agent which gets reduced in the process. The agent that pulls off the proton (H+) from HCl is water. The agent that donates protons (HCl) is an acid, the agent that accepts protons (H2O) is a base. Bases must have lone pair electrons so they can accept a proton.
ANIMATION: Reaction of HCl and H2O.
In the reaction: HCl(aq) + H2O(l) ---> H3O+(aq) + Cl-(aq)
If you look at the products, you could imagine they could also react in a reverse of the original reaction to produce the original reactants.
H3O+ + Cl- ---> HCl(aq) + H2O(l)
In this reaction:
Why doesn't this reverse reaction also occur? (Didn't we discuss this with redox reactions above?) It actually does to an extremely small extent we can barely measure any undissociated HCl. You could actually envision the original reaction as reversible:
HCl(aq)A + H2O(l)B <===> H3O+(aq)A + Cl-(aq)B
where A indicates which reactants/products are potential acids in the forward and reverse reactions, and B indicates potential bases. Which way does this reaction go? We will discuss this in more detail in the next chapter and next semester, but suffice it to say, the reaction goes in the direction from the stronger acid and base to the weaker. In the above example, HCl(aq) is the stronger A and H2O(l) the stronger B. You can't predict from looking at the possibilities, but in the next chapter and next semester we will discuss how you can determine the relative strengths of acids and bases by knowing their Lewis structures and from data in tables. Strong acids such as nitric acid, sulfuric acid, hydrochloric acid are essentially completely ionized - they are strong electrolytes.
The simplest kind of acid base reaction that we experience often in the lab is that of a strong acid with a strong base. For us, the strong bases are those that contain OH- ions. Hence
HCl(aq)A + NaOH(aq)B <===> H2O (l)A + NaCl(aq)B - molecular eq.
H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq)
<===> H2O (l) + Na+(aq) + Cl-(aq)
ionic equation
H+(aq)+ OH-(aq) <===> H2O (l) net ionic equation
Note: a strong acid reacts with a strong base to form a salt and water - the acid and base are neutralized.
SUMMARY ACIDS/BASES
1. Acids are proton donors, bases are proton acceptors.
2. Acids and bases can be strong or weak.
3a. A strong acids interacts completely in water (ionize) to form H3O+ and a negative ion.
Ex. HCl + H2O ----------> H3O+ + Cl-
3b. Strong acids interact completely with a strong base like hydroxide to form a salt and water in a neutralization reaction.
Ex: HCl + NaOH ----------> H2O + NaCl
4. Other strong acids are sulfuric acid, H2SO4, nitric acid, and HNO3
5. Strong acids are strong electrolytes.
6a. Weak acids interact incompletely in water (ionize) to form H3O+ and a negative ion.
Ex. CH3COOH + H2O ----------> H3O+ + CH3COO-
6b. Weak acids interact completely with a strong base like hydroxide to form a salt and water in a neutralization reaction.
Ex: CH3COOH + OH- ----------> H2O + NaCH3COO
7. Weak acids are weak electrolytes.
8a. Strong bases (like oxides) interact completely in water to form OH-.
Ex. O2- (aq) + H2O(l) -------------> OH-(aq) + OH-(aq)
(note: OH- is also a strong base. If it reacts with water, would form water and another hydroxide)
8b. Strong bases interact completely with strong acids like HCl to form a salt and water in a neutralization reaction.
Ex: HCl + NaOH ----------> H2O + NaCl
9. Other strong bases are soluble oxides, like Na2O and soluble hydroxide salts.
10. Strong bases are strong electrolytes.
11a. Weak bases interact incompletely in water (ionize) to form OH- and a positive ion.
Ex. NH3 + H2O ----------> NH4+ + OH-
11b. Weak bases interact completely with a strong acid like HCl to form a salt.
Ex: NH3 + HCl ----------> NH4Cl
12. Weak bases are weak electrolytes.
13. The pH = - log [H3O]+is a measure of the hydronium concentration of an aqueous solution. The lower the pH the more acidic the solution.
| # | 1000 | 100 | 10 | 0.1 | 0.01 | 0.001 |
| scien.not. | 103 | 102 | 101 | 10-1 | 10-2 | 10-3 |
| log | 3 | 2 | 1 | -1 | -2 | -3 |
| -log | -3 | -2 | -1 | 1 | 2 | 3 |
14. A 0.1 M HCl solution has 0.1 M H3O+ and a pH = 1, whereas a 0.1 M acetic acid solution (CH3COOH) has 0.001 M H3O+ (since it is only 1% dissociated) and a pH = 3
15. The Keq of an acid is a mesure of the strength of an
acid. For the general acid reaction with water:
HA(aq) + H2O(l) <==> H3O+ + A-, Keq
= ([H3O+][A-])/([HA][H2O])
For a strong acid, Keq > 1 and for a weak acid, Keq < 1.
16. [H2O] is approximately 55.5 M in a diliute acid
solution, and is essentially constant (since it is present in such great excess).Hence Keq[H2O]
= ([H3O+][A-])/[HA] = Ka.
Ka, the acid constant, is also a constant; for a strong acid, Ka
> 1 and for a weak acid, Ka < 1
Consider the two reactions below in which the concentration of HCl and CH3COOH are each 0.1 M.:
HCl + H2O ----------> H3O+ + Cl-
17. At equilbrium, [H3O+] and [Cl- ]
are both approximately 0.1 M and HCl is effectively 0. In actuality, some HCl is left and
can be shown to be 0.000000001 M = 10-9 M.
Hence Ka = ([H3O+][A-])/[HA] = (10-1)(10-1)/10-9
= 107 >> 1.
CH3COOH + H2O ----------> H3O+ + CH3COO-
18. At equilbrium, [H3O+] and [Cl- ]
are both approximately 0.001 M and CH3COOH is 0.099 M = 9.9 x10-2 M.
Hence Ka = ([H3O+][A-])/[HA] = (10-3)(10-3)/9.9
x 10-2 = 1.01 x 10-5 << 1.
19. Just as pH = -log[H3O]+, pKa = -logKa. In the above examples, the pKa of HCl is -7 while that of acetic acid is approx. 5.
20. The pKa of an acid is a measure of the strength of an acid. It is a constant and does not depend on the concentration of the acid. The lower the pKa, the more stronger the acid.
21. Water can act as both a weak acid and a weak base. It can interact with itself to form the hydronium and hydroxide ion, as shown below:
H2O + H2O ----------> H3O+ + OH-
22. In pure water, [H3O+] and [OH-] are equal (as seen in the equation above) and are each 1x10-7 M. Hence the pH of neutral water is 7.0
23. Acid/base reactions prefer to go from the direction of strong acid/base (unstable, reactive reactants) to weak acid/base (stable, unreactive products)
24. Acid/base reactions can be driven toward products by increasing the concentration of reactants, and driven toward reactants by increasing the concentration of products.
25. The overall driving forces for the reaction hence depends on two features: the intrinsic reactivity of reactants with respect to products (independent of concentration), and reflected in ΔGostab and Keq and relative concentrations of reactants and products and reflected in ΔGconc.
26. Bases have one or more pairs of nonbonded electrons which they use to form a bond to a proton (i.e. to accept a proton).
27. The strengths of acids of similar structure can be determined by comparing the stability of the negatively charged ion formed after the acid donates its proton.
28. Negative ions in which the negative charge is localized on an electronegative atom are more stable than negative ions in which the charge is localized on a less electronegative ion.

29. Likewise, the negatively charged ion is more stable if the ion is larger (i.e. when negative charge on the ion is more "dilute" or delocalized.

30. Also, a negatively charged ion is more stable than a similar ion if the charge on the first ion can be diluted, or delocalized by resonance.
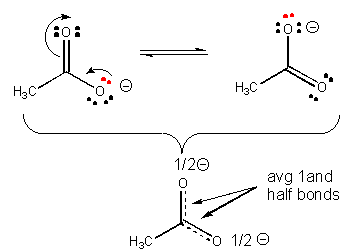
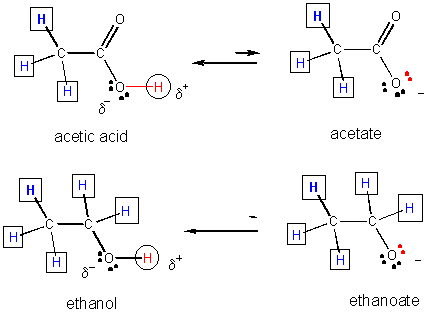
31. Resonance stabilization of the negatively charged product of acetic acid (which is not possible for the anion from ethanol) explains why acetic acid is more acidic than ethanol, as shown below.
BASES
32. Bases are proton acceptors. We have alreay seen two, OH- and H2O, 33. When we compare the strength of these two, it should be clear that OH- is a stronger base since with a negative charge it would be more likely to "accept" a positive proton.
34. Consider an aqueuous solution of Na2O. Such a solution, which consists of sodium and oxide ions, would react with water as shown below:
O2- + H2O --> OH- + OH-
Oxide is a stronger base than OH- since it has two negative charges which should attract a proton more strongly than even OH-. Therefore we can rank these bases from stronger to weaker bases as follows:
O2- > OH- > H2O.
35. Notice also that these bases can all react with water to form OH- in solution.
36. Bases can be strong (like O2- & OH- ) - analogous to strong acids (nitric, sulfuric, hydrochloric) or weak (like H2O and ammonia - NH3).
37. NH3 is a common weak base as shown below. Ammonia accepts a proton from water to form another base, OH-, which is clearly a stronger base than ammonia. Hence, from a stability point of view, the reverse reaction is favored (going from stronger acid and base to weaker acid and base).:
NH3 + H2O --> NH4+ + OH-
38.. Base strength can be detemined by writing relevant acid/base reaction, and knowing which acids are strong or weak. For example, rank the following from least basic to most basic:
Cl-, NO3-, H2O, and CH3COO.
These bases are very different in strucuture. To rank them, write the reactions of acid which produce these bases when they react in water and determine the relative strenght of the bases in each reactions (s = strong, w = weak) :
HCl(
sa) + H2O(sb) <==> H3O+(wa) + Cl-(wb)HNO3(sa) + H2O(sb) <==> H3O+(wa) + NO3-(wa)
CH3COOH(wa) + H2O(wb) <==> H3O+(sa) + CH3COO -(sa)
From the list, clearly, in terms of base strength:
Cl- and NO3- < (less basic) < H2O < CH3COO -
39. For bases that are similiar in structure, the chemical reactivity can be directly compared. Using the following reactions to rank the following from least basic to most basic: F-, Cl-, Br-, I-.
HF + H2O --> H3O+ + F-
HCl + H2O --> H3O+ + Cl-
HBr + H2O --> H3O+ + Br-
Hl + H2O --> H3O+ + I-
We studied this example earlier, where we ranked HI the strongest acid and HF the weakest in the series (due to dilution of the negative charge on the product). Therefore, I- is most stable, and least basic, while F- is least stable and most basic.
WCB Quiz: Acids/Bases - Strength and Stability
WCB Quiz: Acids/Bases - Strength and Stability -ANSWERS
Polyprotic Acids
Some acids have more than one proton attached to an electronegative atom like oxygen. Examples of such oxyacids are:
sulfuric acid - H2SO4: This acid is a strong acid and can donate two protons in the presence of a strong base like hydoxide:
H2SO4 + OH- ---> H2O + HSO4- ; HSO4- + OH- ---> H2O + SO42-
phosphoric acid - H3PO4: This acid is not as strong, and can donate three protons in the presence of a strong base like hydroxide.
H3PO4 + OH- ---> H2O + H2PO4- ; H2PO4- + OH- ---> H2O + HPO42- ;
HPO42- + OH- ---> H2O + PO43-
Since acids are proton donors, each successive proton that is removed from a polyprotic acid should come off with increasing difficulty, since the proton is being removed from a species of increasing negative charge. Hence, it should be clear that with respect to acid strength:
H2SO4 > HSO4-
H3PO4 > H2PO4- > HPO42-
When water, which is much less basic than OH-, interacts with H3PO4 and carbonic acid, H2CO3, another weak acid, not all the protons on these polyprotic acids are donated water. Hence PO43- and CO32- are not found to any extent in a solution of phosphoric acid and carbonic acid. In practical terms, when you put phosphoric acid in water, the first proton essentially dissociates to form H2PO4-. However, H2PO4- is a much weaker acid (pKa = 7.21 - weaker than acetic acid) so little of this dissociates to form HPO42-. This should be no surprise since an even stronger acid - acetic acid - only dissociates to about 1%.
Carbonic acid - H2CO3: This is a weak acid that can react with OH- as follows:
H2CO3 + OH- ---> H2O + HCO3- ; HCO3- + OH- ---> H2O + CO32-
CO32- is the carbonate ion, while HCO3- is bicarbonate ion. Many of you have used sodium bicarbonate, NaHCO3 in the lab to neutralize acid and base spills. Its ability to do this is described in the equations below:
HCO3- + OH- ---> H2O + CO32- (which neutralized OH-)
HCO3- + H3O+ ---> H2O + H2CO3 (which forms a much weaker acid - carbonic acid).
BUFFERS
32. Buffer solutions consist of weak acids and salts of weak acids. They can be created by mixing a solution of a weak acid (such as acetic acid) with a solution of a salt of a weak acid (such as sodium acetate)
33. Alternatively, buffer solutions can be created by partially titrating a solution of a weak acid with sodium hydroxide which neutralizes part of the acid to form the salt of the weak acid, with the sodium ion provided by the added titrant.
34. Buffer solutions can neutralize the addition of small amounts of an acid (which reacts with the base product of the weak acid) and small amounts of a base (which reacts with the weak acid).
Consider what would happen to the pH of pure water (pH = 7) if either 0.01 mol of NaOH or 0.01 mol of HCl were added to sufficient water to make 1L. The first solution would now have an [OH-] = 0.01 M and hence a [H3O+] = 1 x 10-12 M, or a pH = 12. The second solution would have a [H3O+] = 1 x 10-2 M, or a pH = 2. This examples shows that the addition of a small amount fo either acid or base to water dramatically changes the pH. Consider the pH of pure water to be like a balance. Addition of small amounts of either acid or bases dramatically changes the balance (pH)
Animation: Addition of NaOH and HCl to water
Now consider what would happen to the pH of a 0.5 M acetic acid (a weak acid) solution but which also contains 0.5 M sodium acetate (a salt of a weak acid). The appropriate equation to describe this solution is:
CH3COOH + H2O <==> H3O+ + CH3COO-
The pH of the solution can be calculate if you know the Ka of acetic acid = 1.8 x 10-5. Plug this into the following equation to get x = [H3O+] and pH as follows:
Ka = ([H3O+] [CH3COO-])/[CH3COOH] = x(0.5)/0.5 = 1.8 x 10-5
x = [H3O+] = 1.8 x 10-5; pH = 4.74
Now if 0.01 mol of NaOH was added (without changing the volume), the hydroxide would react with the weak acid as shown below:
CH3COOH + OH- <==> H2O + CH3COO-
Therefore the base is consumed, CH3COOH is reduced to 0.49 M and CH3COO- increases to 0.51. As above, x can be calculated and the pH determined.
Ka = ([H3O+] [CH3COO-])/[CH3COOH] = x(0.51)/0.49 = 1.8 x 10-5
x = [H3O+] = 1.72 x 10-5; pH = 4.76.
The pH was raised only by 0.02 units in comparison to the case of pure water whose pH increased by 5 units.
Now if 0.01 mol of HCl was added (without changing the volume), the HCl would react with the weak base acetate as shown below:
HCl + CH3COO- <==> Cl- + CH3COOH
Therefore the added HCl is consumed, CH3COO- is reduced to 0.49 M and CH3COOH increases to 0.51. As above, x can be calculated and the pH determined.
Ka = ([H3O+] [CH3COO-])/[CH3COOH] = x(0.49)/0.51 = 1.8 x 10-5
x = [H3O+] = 1.87 x 10-5; pH = 4.73. The pH was reduced only by 0.01 units in comparison to the case of pure water whose pH dropped by 5 units.
Animation: Addition of NaOH and HCl to an acetic acid/sodium acetate buffer
Biological Buffer Systems:
pH in the body has to be very tightly controlled. Specifically, the pH of blood and intracellular spaces can not very much without serious consequences. Two common buffers systems are
The later is most important in blood, which is maintained within a narrow range of 7.35-7.45. This buffer system is a bit more complicated since another reaction of carbonic acid must be considered - namely the breakdown of carbonic acid to water and CO2.
CO2(g) + H2O(l) <==> H2CO3(aq) + H2O(l) <==> H3O+(aq) + HCO3-(aq)
I demonstrated the reversible formation of carbonic acid in class by blowing into a water which was made slightly alkaline by the addition of a small amount of ammonia. The solution become more acidic, as indicated by the change of color of the solution when phenopthalein was added. The reaction of carbon dioxide with water to form carbon dioxide can be visualized below:

The two major components of the buffer system of blood are controlled by two different mechanisms:
H2CO3 is controlled changing the respiration rate. If you breath more quickly and deeply (like when you hyperventiallate), you exhale more CO2. This will decrease the blood carbonic acid by as shown in blue below.
CO2(g) + H2O(l) <==> H2CO3(aq) + H2O(l) <==> H3O+(aq) + HCO3-(aq)
HCO3-(aq) (bicarbonate) is controlled by the rate at which it is excreted by the urine.
What happens to the blood pH when the relative amounts of H2CO3(aq) and HCO3-(aq) change. Lets examine the Ka for the red part of the equation below, considering carbonic acid as a weak acid.
CO2(g) + H2O(l) <==> H2CO3(aq) + H2O(l) <==> H3O+(aq) + HCO3-(aq)
Ka = ([H3O+][HCO3-])/[H2CO3] which can be rearranged to
[H3O+] = Ka[H2CO3] /[HCO3-]. From this is should be clear that:
Two kinds of acidosis are common - respiratory acidosis and metabolic acidosis.
Metabolic acidosis: This occurs often in burn patients. Blood plasma leaks into damaged areas which can decrease total blood volume and flow, decreasing oxygen availability. This will increase anaerobic metabolism (which we will study next semester), just as when you are running a fast race and are deprived of oxygen. Lactic acid builds up in the blood, increasing the H3O+ in the blood, shifts the equilibrium shown in red below to increase carbonic acid and decrease bicarbonate:
CO2(g) + H2O(l) <==> H2CO3(aq) + H2O(l) <==> H3O+(aq) + HCO3-(aq)
This decreased pH causes the injured person to break harder to get rid of CO2, which by Le Chateliers principle causes a helpful decrease in carbonic acid as shown in blue below:
CO2(g) + H2O(l) <==> H2CO3(aq) + H2O(l) <==> H3O+(aq) + HCO3-(aq)
If the damage is to great so the person can't breath well, shock can develop.
Respiratory acidosis: This occurs in burn victims who have inhaled much smoke and can't breath well. Likewise it occurs in pulmonary diseases such as emphysema or a physical obstruction which hinders respiration. This causes CO2 to build up, increasing carbonic acid and decreasing blood pH as shown below in red:
CO2(g) + H2O(l) <==> H2CO3(aq) + H2O(l) <==> H3O+(aq) + HCO3-(aq)